Bij vele neurodegeneratieve ziekten ontstaan eiwitaggregaten, die dan direct de schuld krijgen van het ziektebeeld. Beter is het om de ware oorzaak van aggregaatvorming te achterhalen. In dit artikel wordt de verklaring bij autofagie gezocht, die een beter celfysiologisch inzicht geeft in de vorming van aggregaten. Enkele voedingsgerelateerde therapieën worden aangehaald.
Beste bezoeker, u heeft geen toegang.
Enkel (web)abonnees hebben toegang tot tijdschriftartikelen. Het webabonnement is nog in de maak.
U kunt zich wel alvast (gratis) registreren en tal van andere webartikelen raadplegen!
Auteur
Verschenen in
Referenties
1. Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014 Jan;24(1):24-41. doi: 10.1038/cr.2013.168. Epub 2013 Dec 24. PMID: 24366339; PMCID: PMC3879710.
https://pubmed.ncbi.nlm.nih.gov/24366339/
2. Mathiassen SG, De Zio D, Cecconi F. Autophagy and the Cell Cycle: A Complex Landscape. Front Oncol. 2017 Mar 31;7:51. doi: 10.3389/fonc.2017.00051. PMID: 28409123; PMCID: PMC5374984.
https://pubmed.ncbi.nlm.nih.gov/28409123/
3. Huang Y, Jiang X, Liang X, Jiang G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol Lett. 2018 May;15(5):6063-6076. doi: 10.3892/ol.2018.8123. Epub 2018 Feb 27. PMID: 29616091; PMCID: PMC5876469.
https://pubmed.ncbi.nlm.nih.gov/29616091/
4. Guo F, Liu X, Cai H, Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. 2018 Jan;28(1):3-13. doi: 10.1111/bpa.12545. Epub 2017 Aug 6. PMID: 28703923; PMCID: PMC5739982.
https://pubmed.ncbi.nlm.nih.gov/28703923/
5. Ichimiya T, Yamakawa T, Hirano T, Yokoyama Y, Hayashi Y, Hirayama D, Wagatsuma K, Itoi T, Nakase H. Autophagy and Autophagy-Related Diseases: A Review. Int J Mol Sci. 2020 Nov 26;21(23):8974. doi: 10.3390/ijms21238974. PMID: 33255983; PMCID: PMC7729615.
https://pubmed.ncbi.nlm.nih.gov/33255983/
6. Tabibzadeh S. Role of autophagy in aging: The good, the bad, and the ugly. Aging Cell. 2023 Jan;22(1):e13753. doi: 10.1111/acel.13753. Epub 2022 Dec 20. PMID: 36539927; PMCID: PMC9835585.
https://pubmed.ncbi.nlm.nih.gov/36539927/
7. Diab R, Pilotto F, Saxena S. Autophagy and neurodegeneration: Unraveling the role of C9ORF72 in the regulation of autophagy and its relationship to ALS-FTD pathology. Front Cell Neurosci. 2023 Mar 16;17:1086895. doi: 10.3389/fncel.2023.1086895. Erratum in: Front Cell Neurosci. 2023 Jun 07;17:1225439. PMID: 37006471; PMCID: PMC10060823.
https://pubmed.ncbi.nlm.nih.gov/37006471/
Autophagy in neurodegenerative diseases
8. Mackenzie IR, Rademakers R. The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia. Curr Opin Neurol. 2008 Dec;21(6):693-700. doi: 10.1097/WCO.0b013e3283168d1d. PMID: 18989115; PMCID: PMC2869081.
https://pubmed.ncbi.nlm.nih.gov/18989115/
9. Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013 Sep 5;501(7465):45-51. doi: 10.1038/nature12481. PMID: 24005412; PMCID: PMC3963807.
https://pubmed.ncbi.nlm.nih.gov/24005412/
10. Tutar, Y., Özgür, A., Tutar, L., Tutar, Y., Özgür, A., & Tutar, L. (2013). Role of protein aggregation in neurodegenerative diseases. Neurodegenerative Diseases, 55-76.
https://books.google.nl/books?hl=nl&lr=&id=8XKfDwAAQBAJ&oi=fnd&pg=PA55&d...
11. Wolfrum P, Fietz A, Schnichels S, Hurst J. The function of p53 and its role in Alzheimer's and Parkinson's disease compared to age-related macular degeneration. Front Neurosci. 2022 Dec 21;16:1029473. doi: 10.3389/fnins.2022.1029473. PMID: 36620455; PMCID: PMC9811148.
https://pubmed.ncbi.nlm.nih.gov/36620455/
12. Frade JM, López-Sánchez N. A novel hypothesis for Alzheimer disease based on neuronal tetraploidy induced by p75 (NTR). Cell Cycle. 2010 May 15;9(10):1934-41. doi: 10.4161/cc.9.10.11582. Epub 2010 May 15. PMID: 20436277.
https://pubmed.ncbi.nlm.nih.gov/20436277/
Transition metals in neurodegenerative diseases
13. Exley, C., & Korchazhkina, O. (2001). The association of aluminium and β amyloid in Alzheimer’s disease. In Aluminium and Alzheimer's Disease (pp. 421-433). Elsevier.
https://scholar.google.nl/scholar?hl=nl&as_sdt=0%2C5&q=Chapter+22+-+The+...
14. Musgrave I. The conversation. Does aluminium cause Alzheimer’s and breast cancer? Published: March 24, 2013 8.04pm CET, accessed 15 august 2023
https://theconversation.com/does-aluminium-cause-alzheimers-and-breast-c...
15. Del Barrio, M., Borghesani, V., Hureau, C., & Faller, P. (2017). Metal-binding to amyloid-β peptide: Coordination, aggregation, and reactive oxygen species production. In Biometals in Neurodegenerative Diseases (pp. 265-281). Academic Press.
https://scholar.google.nl/scholar?hl=nl&as_sdt=0%2C5&q=del+Barrio+Biomet...
16. Lupaescu AV, Humelnicu I, Petre BA, Ciobanu CI, Drochioiu G. Direct evidence for binding of aluminum to NAP anti-amyloid peptide and its analogs. Eur J Mass Spectrom (Chichester). 2020 Apr;26(2):106-116. doi: 10.1177/1469066719877714. Epub 2019 Sep 24. PMID: 31550911.
https://pubmed.ncbi.nlm.nih.gov/31550911/
17. Wiley CD, Campisi J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat Metab. 2021 Oct;3(10):1290-1301. doi: 10.1038/s42255-021-00483-8. Epub 2021 Oct 18. PMID: 34663974; PMCID: PMC8889622.
https://pubmed.ncbi.nlm.nih.gov/34663974/
18. Mold MJ, O'Farrell A, Morris B, Exley C. Aluminum and Tau in Neurofibrillary Tangles in Familial Alzheimer's Disease. J Alzheimers Dis Rep. 2021 Apr 9;5(1):283-294. doi: 10.3233/ADR-210011. PMID: 34113785; PMCID: PMC8150251.
https://pubmed.ncbi.nlm.nih.gov/34113785/
Prion Disease
9. Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013 Sep 5;501(7465):45-51. doi: 10.1038/nature12481. PMID: 24005412; PMCID: PMC3963807.
https://pubmed.ncbi.nlm.nih.gov/24005412/
19. Centers for Disease Control and Prevention (CDC). Variant Creutzfeldt-Jakob Disease (vCJD), accessed 19 august 2023
https://www.cdc.gov/prions/vcjd/factsheet.html#:~:text=and%20Human%20Ser....
Monoclonal antibodies directed against protein aggregates
20. US Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Immediate Release: January 06, 2023, accessed 15 august 2023
https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat...
21. Harris E. Alzheimer Drug Lecanemab Gains Traditional FDA Approval. JAMA. 2023 Aug 8;330(6):495. doi: 10.1001/jama.2023.12548. PMID: 37466999.
https://pubmed.ncbi.nlm.nih.gov/37466999/
21a. Whone A. Monoclonal Antibody Therapy in Parkinson's Disease - The End? N Engl J Med. 2022 Aug 4;387(5):466-467. doi: 10.1056/NEJMe2207681. PMID: 35921458.
https://pubmed.ncbi.nlm.nih.gov/35921458/
21b. Makin S. The amyloid hypothesis on trial. Nature. 2018 Jul;559(7715):S4-S7. doi: 10.1038/d41586-018-05719-4. PMID: 30046080.
https://pubmed.ncbi.nlm.nih.gov/30046080/
22. Eisai Presents Full Results of Lecanemab Phase 3 Confirmatory Clarity Ad Study for Early Alzheimer’s Disease At Clinical Trials On Alzheimer’s Disease (Ctad) Conference, november 29, 2022, accessed 15 august 2023
https://investors.biogen.com/news-releases/news-release-details/eisai-pr...
23. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
https://pubmed.ncbi.nlm.nih.gov/36449413/
24. Alzheimer Nederland. Amerikanen laten alzheimermedicijn Lecanemab toe op de markt. 7 juli 2023, accessed 15 augustus 2023
https://www.alzheimer-nederland.nl/nieuws/amerikanen-laten-alzheimermedi...
Neurological damage
25. Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J Clin Invest. 2005 Jun;115(6):1449-57. doi: 10.1172/JCI24761. PMID: 15931380; PMCID: PMC1137006.
https://pubmed.ncbi.nlm.nih.gov/15931380/
25a. Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35(22):7475-84. doi: 10.1093/nar/gkm744. Epub 2007 Oct 16. PMID: 17942417; PMCID: PMC2190721.
https://pubmed.ncbi.nlm.nih.gov/17942417/
26. Papazoglu C, Mills AA. p53: at the crossroad between cancer and ageing. J Pathol. 2007 Jan;211(2):124-33. doi: 10.1002/path.2086. PMID: 17200941.
https://pubmed.ncbi.nlm.nih.gov/17200941/
27. Zekanowski C, Wojda U. Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer's disease. Acta Neurobiol Exp (Wars). 2009;69(2):232-53. PMID: 19593337.
https://pubmed.ncbi.nlm.nih.gov/19593337/
28. Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021 Feb;22(2):75-95. doi: 10.1038/s41580-020-00314-w. Epub 2020 Dec 16. PMID: 33328614; PMCID: PMC8344376.
https://pubmed.ncbi.nlm.nih.gov/33328614/
29. Wong GC, Chow KH. DNA Damage Response-Associated Cell Cycle Re-Entry and Neuronal Senescence in Brain Aging and Alzheimer's Disease. J Alzheimers Dis. 2023;94(s1):S429-S451. doi: 10.3233/JAD-220203. PMID: 35848025.
https://pubmed.ncbi.nlm.nih.gov/35848025/
Apolipoprotein E4: Alzheimer and heart disease risk
30. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921-3. doi: 10.1126/science.8346443. PMID: 8346443.
https://pubmed.ncbi.nlm.nih.gov/8346443/
30a. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, Huentelman MJ, Beach TG, Caselli RJ, Chen Y, Su Y, Myers AJ, Hardy J, Paul Vonsattel J, Younkin SG, Bennett DA, De Jager PL, Larson EB, Crane PK, Keene CD, Kamboh MI, Kofler JK, Duque L, Gilbert JR, Gwirtsman HE, Buxbaum JD, Dickson DW, Frosch MP, Ghetti BF, Lunetta KL, Wang LS, Hyman BT, Kukull WA, Foroud T, Haines JL, Mayeux RP, Pericak-Vance MA, Schneider JA, Trojanowski JQ, Farrer LA, Schellenberg GD, Beecham GW, Montine TJ, Jun GR; Alzheimer’s Disease Genetics Consortium. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020 Feb 3;11(1):667. doi: 10.1038/s41467-019-14279-8. PMID: 32015339; PMCID: PMC6997393.
https://pubmed.ncbi.nlm.nih.gov/32015339/
30b. APOE Alzheimer's Risk TEST: 504040 Test number copied CPT: 81401, , accessed 2 september 2023
https://www.labcorp.com/tests/504040/i-apoe-i-alzheimer-s-risk
30c. Is Alzheimer's Genetic? Alzheimer’s Association, accessed 2 september 2023
https://www.alz.org/alzheimers-dementia/what-is-alzheimers/causes-and-ri...
30d. McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, Fontenot MB, Bradley BJ. The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes). PLoS One. 2012;7(10):e47760. doi: 10.1371/journal.pone.0047760. Epub 2012 Oct 24. PMID: 23112842; PMCID: PMC3480407.
https://pubmed.ncbi.nlm.nih.gov/23112842/
30e. Iacono D, Feltis GC. Impact of Apolipoprotein E gene polymorphism during normal and pathological conditions of the brain across the lifespan. Aging (Albany NY). 2019 Jan 24;11(2):787-816. doi: 10.18632/aging.101757. PMID: 30677746; PMCID: PMC6366964.
https://pubmed.ncbi.nlm.nih.gov/30677746/
30f. Trumble BC, Charifson M, Kraft T, Garcia AR, Cummings DK, Hooper P, Lea AJ, Eid Rodriguez D, Koebele SV, Buetow K, Beheim B, Minocher R, Gutierrez M, Thomas GS, Gatz M, Stieglitz J, Finch CE, Kaplan H, Gurven M. Apolipoprotein-ε4 is associated with higher fecundity in a natural fertility population. Sci Adv. 2023 Aug 9;9(32):eade9797. doi: 10.1126/sciadv.ade9797. Epub 2023 Aug 9. PMID: 37556539; PMCID: PMC10411886.
https://pubmed.ncbi.nlm.nih.gov/37556539/
31. Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004 Jul 20;141(2):137-47. doi: 10.7326/0003-4819-141-2-200407200-00013. PMID: 15262670.
https://pubmed.ncbi.nlm.nih.gov/15262670/
32. Reiman EM, Arboleda-Velasquez JF, Quiroz YT, Huentelman MJ, Beach TG, Caselli RJ, Chen Y, Su Y, Myers AJ, Hardy J, Paul Vonsattel J, Younkin SG, Bennett DA, De Jager PL, Larson EB, Crane PK, Keene CD, Kamboh MI, Kofler JK, Duque L, Gilbert JR, Gwirtsman HE, Buxbaum JD, Dickson DW, Frosch MP, Ghetti BF, Lunetta KL, Wang LS, Hyman BT, Kukull WA, Foroud T, Haines JL, Mayeux RP, Pericak-Vance MA, Schneider JA, Trojanowski JQ, Farrer LA, Schellenberg GD, Beecham GW, Montine TJ, Jun GR; Alzheimer’s Disease Genetics Consortium. Exceptionally low likelihood of Alzheimer's dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun. 2020 Feb 3;11(1):667. doi: 10.1038/s41467-019-14279-8. PMID: 32015339; PMCID: PMC6997393.
https://pubmed.ncbi.nlm.nih.gov/32015339/
33. Xu M, Zhao J, Zhang Y, Ma X, Dai Q, Zhi H, Wang B, Wang L. Apolipoprotein E Gene Variants and Risk of Coronary Heart Disease: A Meta-Analysis. Biomed Res Int. 2016;2016:3912175. doi: 10.1155/2016/3912175. Epub 2016 Oct 27. PMID: 27868062; PMCID: PMC5102878.
https://pubmed.ncbi.nlm.nih.gov/27868062/
Risk factors for cardiovascular disease and dementia, and the epidemiology of dementia
34. Korczyn AD, Vakhapova V. The prevention of the dementia epidemic. J Neurol Sci. 2007 Jun 15;257(1-2):2-4. doi: 10.1016/j.jns.2007.01.081. Epub 2007 May 8. PMID: 17490685.
https://pubmed.ncbi.nlm.nih.gov/17490685/
34a. Kroner Z. The relationship between Alzheimer's disease and diabetes: Type 3 diabetes? Altern Med Rev. 2009 Dec;14(4):373-9. PMID: 20030463.
https://pubmed.ncbi.nlm.nih.gov/20030463/
35. Leszek J, Mikhaylenko EV, Belousov DM, Koutsouraki E, Szczechowiak K, Kobusiak-Prokopowicz M, Mysiak A, Diniz BS, Somasundaram SG, Kirkland CE, Aliev G. The Links between Cardiovascular Diseases and Alzheimer's Disease. Curr Neuropharmacol. 2021;19(2):152-169. doi: 10.2174/1570159X18666200729093724. PMID: 32727331; PMCID: PMC8033981.
https://pubmed.ncbi.nlm.nih.gov/32727331/
36. Brain, J., Greene, L., Tang, E. Y., Louise, J., Salter, A., Beach, S., & Tully, P. J. (2023). Cardiovascular disease, associated risk factors, and risk of dementia: An umbrella review of meta-analyses. Frontiers in Epidemiology, 3, 1095236.
https://www.frontiersin.org/articles/10.3389/fepid.2023.1095236/full
36a. Alzheimer Nederland. Factsheet cijfers en feiten over dementia. Bekijk de factsheet met feiten over dementia, accessed 19 augustus 2023
https://www.alzheimer-nederland.nl/factsheet-cijfers-en-feiten-over-deme...
36b. Top 10 doodsoorzaken in Nederland. Verpleeg collectief, wo, 07/19/2017 - 12:06, accessed 21 august 2023
https://verpleegcollectief.nl/particuliere-thuiszorg/dementie/hulp-bij-d...
36c. Nordestgaard LT, Christoffersen M, Frikke-Schmidt R. Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease. Int J Mol Sci. 2022 Aug 29;23(17):9777. doi: 10.3390/ijms23179777. PMID: 36077172; PMCID: PMC9456552.
https://pubmed.ncbi.nlm.nih.gov/36077172/
36d. Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, Rosa GM. Alzheimer's Disease and Cardiovascular Disease: A Particular Association. Cardiol Res Pract. 2020 May 5;2020:2617970. doi: 10.1155/2020/2617970. PMID: 32454996; PMCID: PMC7222603.
https://pubmed.ncbi.nlm.nih.gov/32454996/
Parkinson and pesticides
37. Moisan F, Spinosi J, Delabre L, Gourlet V, Mazurie JL, Bénatru I, Goldberg M, Weisskopf MG, Imbernon E, Tzourio C, Elbaz A. Association of Parkinson's Disease and Its Subtypes with Agricultural Pesticide Exposures in Men: A Case-Control Study in France. Environ Health Perspect. 2015 Nov;123(11):1123-9. doi: 10.1289/ehp.1307970. Epub 2015 Mar 27. PMID: 25815770; PMCID: PMC4629732.
https://pubmed.ncbi.nlm.nih.gov/25815770/
38. Yan D, Zhang Y, Liu L, Shi N, Yan H. Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol. 2018 Jul;96:57-63. doi: 10.1016/j.yrtph.2018.05.005. Epub 2018 May 3. PMID: 29729297.
https://pubmed.ncbi.nlm.nih.gov/29729297/
39. Leszek J, Mikhaylenko EV, Belousov DM, Koutsouraki E, Szczechowiak K, Kobusiak-Prokopowicz M, Mysiak A, Diniz BS, Somasundaram SG, Kirkland CE, Aliev G. The Links between Cardiovascular Diseases and Alzheimer's Disease. Curr Neuropharmacol. 2021;19(2):152-169. doi: 10.2174/1570159X18666200729093724. PMID: 32727331; PMCID: PMC8033981.
https://pubmed.ncbi.nlm.nih.gov/32727331/
Cell cycle arrest
40. Yurov YB, Vorsanova SG, Iourov IY. The DNA replication stress hypothesis of Alzheimer's disease. ScientificWorldJournal. 2011;11:2602-12. doi: 10.1100/2011/625690. Epub 2012 Jan 2. PMID: 22262948; PMCID: PMC3254013.
https://pubmed.ncbi.nlm.nih.gov/22262948/
41. Wang W, Bu B, Xie M, Zhang M, Yu Z, Tao D. Neural cell cycle dysregulation and central nervous system diseases. Prog Neurobiol. 2009 Sep;89(1):1-17. doi: 10.1016/j.pneurobio.2009.01.007. Epub 2009 Feb 4. PMID: 19619927.
https://pubmed.ncbi.nlm.nih.gov/19619927/
42. Palsson-McDermott EM, O'Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013 Nov;35(11):965-73. doi: 10.1002/bies.201300084. Epub 2013 Sep 20. PMID: 24115022.
https://pubmed.ncbi.nlm.nih.gov/24115022/
43. Kee HJ, Cheong JH. Tumor bioenergetics: an emerging avenue for cancer metabolism targeted therapy. BMB Rep. 2014 Mar;47(3):158-66. doi: 10.5483/bmbrep.2014.47.3.273. PMID: 24499670; PMCID: PMC4163877.
https://pubmed.ncbi.nlm.nih.gov/24499670/
44. Icard P, Fournel L, Wu Z, Alifano M, Lincet H. Interconnection between Metabolism and Cell Cycle in Cancer. Trends Biochem Sci. 2019 Jun;44(6):490-501. doi: 10.1016/j.tibs.2018.12.007. Epub 2019 Jan 14. PMID: 30655165.
https://pubmed.ncbi.nlm.nih.gov/30655165/
45. Moh C, Kubiak JZ, Bajic VP, Zhu X, Smith MA, Lee HG. Cell cycle deregulation in the neurons of Alzheimer's disease. Results Probl Cell Differ. 2011;53:565-76. doi: 10.1007/978-3-642-19065-0_23. PMID: 21630160; PMCID: PMC5925746.
https://pubmed.ncbi.nlm.nih.gov/21630160/
46. Yang S, Shin J, Park KH, Jeung HC, Rha SY, Noh SH, Yang WI, Chung HC. Molecular basis of the differences between normal and tumor tissues of gastric cancer. Biochim Biophys Acta. 2007 Sep;1772(9):1033-40. doi: 10.1016/j.bbadis.2007.05.005. Epub 2007 May 31. PMID: 17601708.
https://pubmed.ncbi.nlm.nih.gov/17601708/
47. Barrio-Alonso E, Hernández-Vivanco A, Walton CC, Perea G, Frade JM. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci Rep. 2018 Sep 25;8(1):14316. doi: 10.1038/s41598-018-32708-4. PMID: 30254284; PMCID: PMC6156334.
https://pubmed.ncbi.nlm.nih.gov/30254284/
Warburg effect
47a. Muskiet FAJ. Het Warburgeffect is overal. Uitzicht 2022, 47 no 3, pagina 43.
https://www.mmv.nl/nieuws/het-warburgeffect-is-overal/
47b. Muskiet FAJ. Het Warburg effect, een rode draad door kanker en chronische degeneratieve ziektes. Voedingsgeneeskunde 2022;23(3)58-59.
https://www.voedingsgeneeskunde.nl/vg-23-6/het-warburgeffect-een-rode-dr...
Senescent cells
48. Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY). 2011 Dec;3(12):1130-41. doi: 10.18632/aging.100422. PMID: 22246147; PMCID: PMC3273893.
https://pubmed.ncbi.nlm.nih.gov/22246147/
49. Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 2016 May 24;114(11):1180-4. doi: 10.1038/bjc.2016.115. Epub 2016 May 3. PMID: 27140310; PMCID: PMC4891501.
https://pubmed.ncbi.nlm.nih.gov/27140310/
50. Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018 Apr 2;128(4):1208-1216. doi: 10.1172/JCI95145. Epub 2018 Feb 19. PMID: 29457783; PMCID: PMC5873891.
https://pubmed.ncbi.nlm.nih.gov/29457783/
51. Wilkinson HN, Hardman MJ. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front Cell Dev Biol. 2020 Aug 11;8:773. doi: 10.3389/fcell.2020.00773. PMID: 32850866; PMCID: PMC7431694.
https://pubmed.ncbi.nlm.nih.gov/32850866/
52. Si Z, Sun L, Wang X. Evidence and perspectives of cell senescence in neurodegenerative diseases. Biomed Pharmacother. 2021 May;137:111327. doi: 10.1016/j.biopha.2021.111327. Epub 2021 Feb 3. PMID: 33545662.
https://pubmed.ncbi.nlm.nih.gov/33545662/
53. Zhang L, Pitcher LE, Yousefzadeh MJ, Niedernhofer LJ, Robbins PD, Zhu Y. Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest. 2022 Aug 1;132(15):e158450. doi: 10.1172/JCI158450. PMID: 35912854; PMCID: PMC9337830.
https://pubmed.ncbi.nlm.nih.gov/35912854/
54. Kowald A, Passos JF, Kirkwood TBL. On the evolution of cellular senescence. Aging Cell. 2020 Dec;19(12):e13270. doi: 10.1111/acel.13270. Epub 2020 Nov 9. PMID: 33166065; PMCID: PMC7744960.
https://pubmed.ncbi.nlm.nih.gov/33166065/
54a. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685-705. doi: 10.1146/annurev-physiol-030212-183653. Epub 2012 Nov 8. PMID: 23140366; PMCID: PMC4166529.
https://pubmed.ncbi.nlm.nih.gov/23140366/
High metabolic activity of senescent cells
55. Sabbatinelli J, Prattichizzo F, Olivieri F, Procopio AD, Rippo MR, Giuliani A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front Physiol. 2019 Dec 18;10:1523. doi: 10.3389/fphys.2019.01523. PMID: 31920721; PMCID: PMC6930181.
https://pubmed.ncbi.nlm.nih.gov/31920721/
56. Frasca D, Saada YB, Garcia D, Friguet B. Effects of cellular senescence on metabolic pathways in non-immune and immune cells. Mech Ageing Dev. 2021 Mar;194:111428. doi: 10.1016/j.mad.2020.111428. Epub 2020 Dec 28. PMID: 33383073; PMCID: PMC7882031.
https://pubmed.ncbi.nlm.nih.gov/33383073/
57. Gasek NS, Kuchel GA, Kirkland JL, Xu M. Strategies for Targeting Senescent Cells in Human Disease. Nat Aging. 2021 Oct;1(10):870-879. doi: 10.1038/s43587-021-00121-8. Epub 2021 Oct 7. PMID: 34841261; PMCID: PMC8612694.
https://pubmed.ncbi.nlm.nih.gov/34841261/
58. Dou, X., Long, Q., Liu, S., Zou, Y., Fu, D., Chen, X. & Sun, Y. (2022). Senescent cells develop PDK4-dependent hypercatabolism and form an acidic microenvironment to drive cancer resistance. BioRxiv, 2022-08.
https://www.biorxiv.org/content/10.1101/2022.08.29.505761v1.abstract
Polyploidy of senescent cells
59. Herrup K. The involvement of cell cycle events in the pathogenesis of Alzheimer's disease. Alzheimers Res Ther. 2010 May 20;2(3):13. doi: 10.1186/alzrt37. PMID: 20497605; PMCID: PMC2919693.
https://pubmed.ncbi.nlm.nih.gov/20497605/
60. Nandakumar S, Rozich E, Buttitta L. Cell Cycle Re-entry in the Nervous System: From Polyploidy to Neurodegeneration. Front Cell Dev Biol. 2021 Jun 24;9:698661. doi: 10.3389/fcell.2021.698661. PMID: 34249947; PMCID: PMC8264763.
https://pubmed.ncbi.nlm.nih.gov/34249947/
The senescence-associated secretory phenotype (SASP)
61. Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. doi: 10.1146/annurev-pathol-121808-102144. PMID: 20078217; PMCID: PMC4166495.
https://pubmed.ncbi.nlm.nih.gov/20078217/
62. van Deursen JM. The role of senescent cells in ageing. Nature. 2014 May 22;509(7501):439-46. doi: 10.1038/nature13193. PMID: 24848057; PMCID: PMC4214092.
https://pubmed.ncbi.nlm.nih.gov/24848057/
63. Prieto LI, Baker DJ. Cellular Senescence and the Immune System in Cancer. Gerontology. 2019;65(5):505-512. doi: 10.1159/000500683. Epub 2019 Jun 18. PMID: 31212284; PMCID: PMC6703936.
https://pubmed.ncbi.nlm.nih.gov/31212284/
64. Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider NA, Olayiwola JO, Murray LB, Vinson BT, Chrisey DB, Lord CJ, Jackson JG. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J Cell Biol. 2019 Nov 4;218(11):3827-3844. doi: 10.1083/jcb.201904051. Epub 2019 Sep 17. PMID: 31530580; PMCID: PMC6829672.
https://pubmed.ncbi.nlm.nih.gov/31530580/
65. Napoli M, Flores ER. Beware of thy neighbor: Senescent cancer cells feast on adjacent cells to persist. J Cell Biol. 2019 Nov 4;218(11):3535-3536. doi: 10.1083/jcb.201910040. Epub 2019 Oct 18. PMID: 31628158; PMCID: PMC6829664.
https://pubmed.ncbi.nlm.nih.gov/31628158/
66. Chapman J, Fielder E, Passos JF. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 2019 Jul;593(13):1566-1579. doi: 10.1002/1873-3468.13498. Epub 2019 Jun 27. PMID: 31211858.
https://pubmed.ncbi.nlm.nih.gov/31211858/
Chronic energy (glucose) deficit of senescent brain cells
67. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, Croteau E, Bocti C, Fulop T, Castellano CA. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer's Disease. Front Mol Neurosci. 2016 Jul 8;9:53. doi: 10.3389/fnmol.2016.00053. PMID: 27458340; PMCID: PMC4937039.
https://pubmed.ncbi.nlm.nih.gov/27458340/
68. Butterfield DA, Favia M, Spera I, Campanella A, Lanza M, Castegna A. Metabolic Features of Brain Function with Relevance to Clinical Features of Alzheimer and Parkinson Diseases. Molecules. 2022 Jan 30;27(3):951. doi: 10.3390/molecules27030951. PMID: 35164216; PMCID: PMC8839962.
https://pubmed.ncbi.nlm.nih.gov/35164216/
Downregulation of autophagy during the cell cycle (mitosis)
69. Hanamata S, Kurusu T, Kuchitsu K. Cell Cycle-Dependence of Autophagic Activity and Inhibition of Autophagosome Formation at M Phase in Tobacco BY-2 Cells. Int J Mol Sci. 2020 Dec 1;21(23):9166. doi: 10.3390/ijms21239166. PMID: 33271936; PMCID: PMC7730373.
https://pubmed.ncbi.nlm.nih.gov/33271936/
70. Willson J. Mitosis flips the switch on autophagy control. Nat Rev Mol Cell Biol. 2020 Jan;21(1):4-5. doi: 10.1038/s41580-019-0196-1. PMID: 31758162.
https://pubmed.ncbi.nlm.nih.gov/31758162/
71. Nowosad A, Besson A. Lysosomes at the Crossroads of Cell Metabolism, Cell Cycle, and Stemness. Int J Mol Sci. 2022 Feb 18;23(4):2290. doi: 10.3390/ijms23042290. PMID: 35216401; PMCID: PMC8879101.
https://pubmed.ncbi.nlm.nih.gov/35216401/
72. Almacellas E, Mauvezin C. Emerging roles of mitotic autophagy. J Cell Sci. 2022 Jun 1;135(11):jcs255802. doi: 10.1242/jcs.255802. Epub 2022 Jun 10. PMID: 35686549.
https://pubmed.ncbi.nlm.nih.gov/35686549/
Autophagy in Alzheimer
73. Son JH, Shim JH, Kim KH, Ha JY, Han JY. Neuronal autophagy and neurodegenerative diseases. Exp Mol Med. 2012 Feb 29;44(2):89-98. doi: 10.3858/emm.2012.44.2.031. PMID: 22257884; PMCID: PMC3296817.
https://pubmed.ncbi.nlm.nih.gov/22257884/
74. Lee W, Kim SH. Autophagy at synapses in neurodegenerative diseases. Arch Pharm Res. 2019 May;42(5):407-415. doi: 10.1007/s12272-019-01148-7. Epub 2019 Apr 1. PMID: 30937842.
https://pubmed.ncbi.nlm.nih.gov/30937842/
75. Busser J, Geldmacher DS, Herrup K. Ectopic cell cycle proteins predict the sites of neuronal cell death in Alzheimer's disease brain. J Neurosci. 1998 Apr 15;18(8):2801-7. doi: 10.1523/JNEUROSCI.18-08-02801.1998. PMID: 9525997; PMCID: PMC6792587.
https://pubmed.ncbi.nlm.nih.gov/9525997/
Fasting and exercise boost autophagy
76. Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010 Aug;6(6):702-10. doi: 10.4161/auto.6.6.12376. Epub 2010 Aug 14. PMID: 20534972; PMCID: PMC3106288.
https://pubmed.ncbi.nlm.nih.gov/20534972/
77. Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, Rubinsztein DC, Partridge L, Kroemer G, Labbadia J, Fang EF. Autophagy in healthy aging and disease. Nat Aging. 2021 Aug;1(8):634-650. doi: 10.1038/s43587-021-00098-4. Epub 2021 Aug 12. PMID: 34901876; PMCID: PMC8659158.
https://pubmed.ncbi.nlm.nih.gov/34901876/
Ketones in Alzheimer’s disease
78. Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic Diet in Alzheimer's Disease. Int J Mol Sci. 2019 Aug 9;20(16):3892. doi: 10.3390/ijms20163892. PMID: 31405021; PMCID: PMC6720297.
https://pubmed.ncbi.nlm.nih.gov/31405021/
79. Stubbs BJ, Koutnik AP, Goldberg EL, Upadhyay V, Turnbaugh PJ, Verdin E, Newman JC. Investigating Ketone Bodies as Immunometabolic Countermeasures against Respiratory Viral Infections. Med. 2020 Dec 18;1(1):43-65. doi: 10.1016/j.medj.2020.06.008. Epub 2020 Jul 15. PMID: 32838361; PMCID: PMC7362813.
https://pubmed.ncbi.nlm.nih.gov/32838361/
80. Snyder J, Zhai R, Lackey AI, Sato PY. Changes in Myocardial Metabolism Preceding Sudden Cardiac Death. Front Physiol. 2020 Jun 16;11:640. doi: 10.3389/fphys.2020.00640. PMID: 32612538; PMCID: PMC7308560.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7308560/
81. Hirschberger S, Gellert L, Effinger D, Muenchhoff M, Herrmann M, Briegel JM, Zwißler B, Kreth S. Ketone Bodies Improve Human CD8+ Cytotoxic T-Cell Immune Response During COVID-19 Infection. Front Med (Lausanne). 2022 Jun 16;9:923502. doi: 10.3389/fmed.2022.923502. PMID: 35783654; PMCID: PMC9243504.
https://pubmed.ncbi.nlm.nih.gov/35783654/
82. Jensen NJ, Wodschow HZ, Nilsson M, Rungby J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int J Mol Sci. 2020 Nov 20;21(22):8767. doi: 10.3390/ijms21228767. PMID: 33233502; PMCID: PMC7699472.
https://pubmed.ncbi.nlm.nih.gov/33233502/
83. Yurista SR, Chong CR, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic Potential of Ketone Bodies for Patients With Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021 Apr 6;77(13):1660-1669. doi: 10.1016/j.jacc.2020.12.065. Epub 2021 Feb 23. PMID: 33637354.
https://pubmed.ncbi.nlm.nih.gov/33637354/
84. Pietrzak D, Kasperek K, Rękawek P, Piątkowska-Chmiel I. The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients. 2022 May 6;14(9):1952. doi: 10.3390/nu14091952. PMID: 35565918; PMCID: PMC9102882.
https://pubmed.ncbi.nlm.nih.gov/35565918/
84a. Muskiet FAJ. Ketogeen dieet: de nieuwe Haarlemmerolie? Voedingsgeneeskunde 2022;23(5)54-55
https://www.voedingsgeneeskunde.nl/vg-23-5/ketogeen-dieet
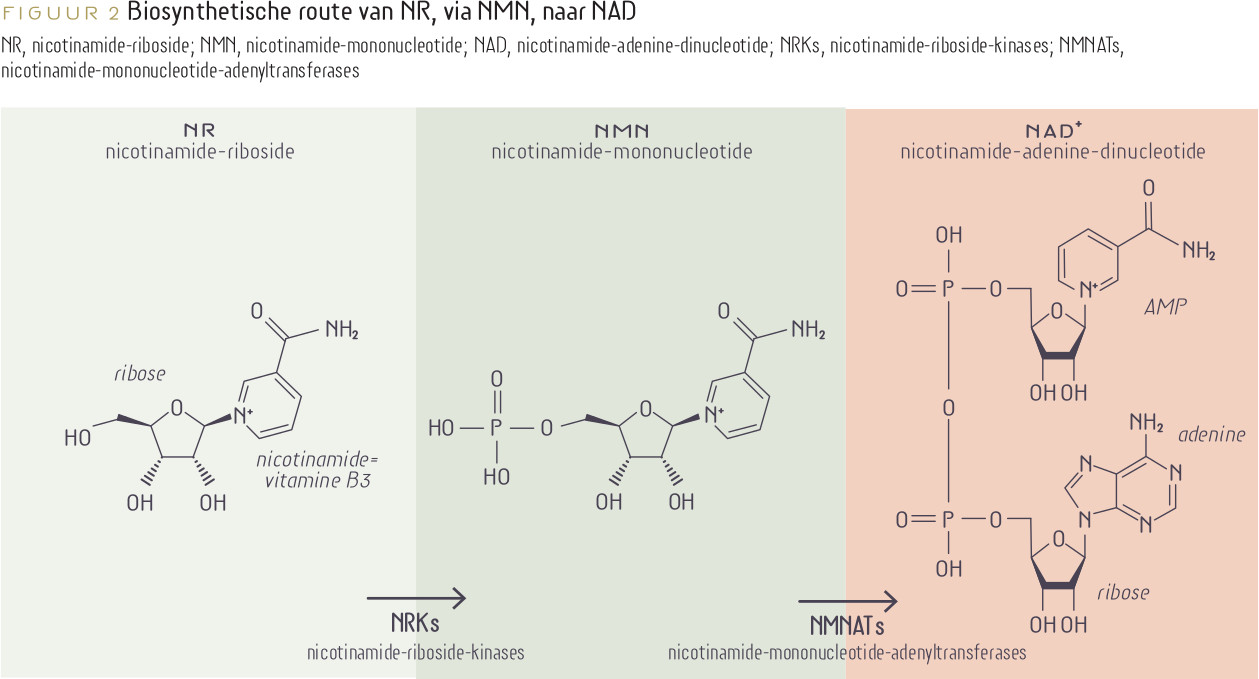
NAD boosting via supplements
85. Dolivo D, Hernandez S, Dominko T. Cellular lifespan and senescence: a complex balance between multiple cellular pathways. Bioessays. 2016 Jul;38 Suppl 1:S33-44. doi: 10.1002/bies.201670906. PMID: 27417120.
https://pubmed.ncbi.nlm.nih.gov/27417120/
85a. Rajman L, Chwalek K, Sinclair DA. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018 Mar 6;27(3):529-547. doi: 10.1016/j.cmet.2018.02.011. PMID: 29514064; PMCID: PMC6342515.
https://pubmed.ncbi.nlm.nih.gov/29514064/
85b. Yoshino J, Baur JA, Imai SI. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018 Mar 6;27(3):513-528. doi: 10.1016/j.cmet.2017.11.002. Epub 2017 Dec 14. PMID: 29249689; PMCID: PMC5842119.
https://pubmed.ncbi.nlm.nih.gov/29249689/
85c. Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020 Oct 7;5(1):227. doi: 10.1038/s41392-020-00311-7. PMID: 33028824; PMCID: PMC7539288.
https://pubmed.ncbi.nlm.nih.gov/33028824/
85d. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021 Feb;22(2):119-141. doi: 10.1038/s41580-020-00313-x. Epub 2020 Dec 22. PMID: 33353981; PMCID: PMC7963035.
https://pubmed.ncbi.nlm.nih.gov/33353981/
85e. Strømland Ø, Diab J, Ferrario E, Sverkeli LJ, Ziegler M. The balance between NAD+ biosynthesis and consumption in ageing. Mech Ageing Dev. 2021 Oct;199:111569. doi: 10.1016/j.mad.2021.111569. Epub 2021 Sep 9. PMID: 34509469.
https://pubmed.ncbi.nlm.nih.gov/34509469/
85f. Abdellatif M, Sedej S, Kroemer G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation. 2021 Nov 30;144(22):1795-1817. doi: 10.1161/CIRCULATIONAHA.121.056589. Epub 2021 Nov 29. PMID: 34843394.
https://pubmed.ncbi.nlm.nih.gov/34843394/
85g. Ur Rahman S, Qadeer A, Wu Z. Role and Potential Mechanisms of Nicotinamide Mononucleotide in Aging. Aging Dis. 2023 Jul 27. doi: 10.14336/AD.2023.0519-1. Epub ahead of print. PMID: 37548938.
https://pubmed.ncbi.nlm.nih.gov/37548938/
85h. Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020 Oct 7;5(1):227. doi: 10.1038/s41392-020-00311-7. PMID: 33028824; PMCID: PMC7539288.
https://pubmed.ncbi.nlm.nih.gov/33028824/
86. Di Micco R, Krizhanovsky V, Baker D, d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021 Feb;22(2):75-95. doi: 10.1038/s41580-020-00314-w. Epub 2020 Dec 16. PMID: 33328614; PMCID: PMC8344376.
https://pubmed.ncbi.nlm.nih.gov/33328614/
87. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac Energy Metabolism in Heart Failure. Circ Res. 2021 May 14;128(10):1487-1513. doi: 10.1161/CIRCRESAHA.121.318241. Epub 2021 May 13. PMID: 33983836; PMCID: PMC8136750.
https://pubmed.ncbi.nlm.nih.gov/33983836/
88. Zheng M, Schultz MB, Sinclair DA. NAD+ in COVID-19 and viral infections. Trends Immunol. 2022 Apr;43(4):283-295. doi: 10.1016/j.it.2022.02.001. Epub 2022 Feb 11. PMID: 35221228; PMCID: PMC8831132.
https://pubmed.ncbi.nlm.nih.gov/35221228/
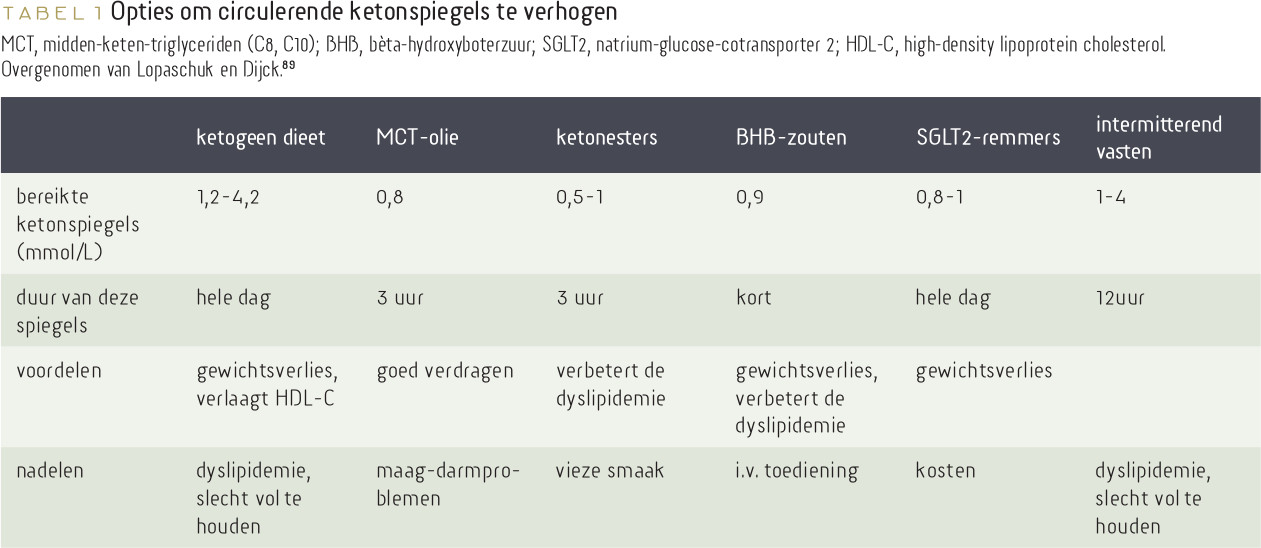
89. Lopaschuk, G. D., & Dyck, J. R. (2023). Ketones and the cardiovascular system. Nature Cardiovascular Research, 1-13.
https://www.nature.com/articles/s44161-023-00259-1
90. Medeiros HCD, Lunt SY. The Warburg effect: Saturation of mitochondrial NADH shuttles triggers aerobic lactate fermentation. Mol Cell. 2022 Sep 1;82(17):3119-3121. doi: 10.1016/j.molcel.2022.08.004. PMID: 36055204; PMCID: PMC9888598.
https://pubmed.ncbi.nlm.nih.gov/36055204/
91. Abdellatif M, Sedej S, Kroemer G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation. 2021 Nov 30;144(22):1795-1817. doi: 10.1161/CIRCULATIONAHA.121.056589. Epub 2021 Nov 29. PMID: 34843394.
https://pubmed.ncbi.nlm.nih.gov/34843394/
92. Victor Ciardha. Researchers Show NMN Improves Cognitive Deficits in Alzheimer’s. The Official Info Source for Nicotinamide Mononucleotide. PST Aug 26, 2022, accessed 15 August 2023
https://www.nmn.com/news/nmn-improves-cognitive-deficits-alzheimers
93. Hu Y, Huang Y, Xing S, Chen C, Shen D, Chen J. Aβ promotes CD38 expression in senescent microglia in Alzheimer's disease. Biol Res. 2022 Mar 3;55(1):10. doi: 10.1186/s40659-022-00379-1. PMID: 35241173; PMCID: PMC8892694.
https://pubmed.ncbi.nlm.nih.gov/35241173/
94. Soma M, Lalam SK. The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Mol Biol Rep. 2022 Oct;49(10):9737-9748. doi: 10.1007/s11033-022-07459-1. Epub 2022 Apr 20. PMID: 35441939.
https://pubmed.ncbi.nlm.nih.gov/35441939/
94a. Zhou Q, Zhu L, Qiu W, Liu Y, Yang F, Chen W, Xu R. Nicotinamide Riboside Enhances Mitochondrial Proteostasis and Adult Neurogenesis through Activation of Mitochondrial Unfolded Protein Response Signaling in the Brain of ALS SOD1G93A Mice. Int J Biol Sci. 2020 Jan 1;16(2):284-297. doi: 10.7150/ijbs.38487. Erratum in: Int J Biol Sci. 2022 Mar 3;18(5):2181-2183. PMID: 31929756; PMCID: PMC6949147.
https://pubmed.ncbi.nlm.nih.gov/31929756/
94b. Brakedal B, Dölle C, Riemer F, Ma Y, Nido GS, Skeie GO, Craven AR, Schwarzlmüller T, Brekke N, Diab J, Sverkeli L, Skjeie V, Varhaug K, Tysnes OB, Peng S, Haugarvoll K, Ziegler M, Grüner R, Eidelberg D, Tzoulis C. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease. Cell Metab. 2022 Mar 1;34(3):396-407.e6. doi: 10.1016/j.cmet.2022.02.001. PMID: 35235774.
https://pubmed.ncbi.nlm.nih.gov/35235774/
94c. Wu W, Yuan S, Tang Y, Meng X, Peng M, Hu Z, Liu W. Effect of Exercise and Oral Niacinamide Mononucleotide on Improving Mitochondrial Autophagy in Alzheimer's Disease. Nutrients. 2023 Jun 23;15(13):2851. doi: 10.3390/nu15132851. PMID: 37447179; PMCID: PMC10343931.
https://pubmed.ncbi.nlm.nih.gov/37447179/
94d. Alegre GFS, Pastore GM. NAD+ Precursors Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR): Potential Dietary Contribution to Health. Curr Nutr Rep. 2023 Sep;12(3):445-464. doi: 10.1007/s13668-023-00475-y. Epub 2023 Jun 5. PMID: 37273100; PMCID: PMC10240123.
https://pubmed.ncbi.nlm.nih.gov/37273100/
94e. Campbell JM. Supplementation with NAD+ and Its Precursors to Prevent Cognitive Decline across Disease Contexts. Nutrients. 2022 Aug 7;14(15):3231. doi: 10.3390/nu14153231. PMID: 35956406; PMCID: PMC9370773.
https://pubmed.ncbi.nlm.nih.gov/35956406/
Stimulation of autophagy
95. Meng T, Lin S, Zhuang H, Huang H, He Z, Hu Y, Gong Q, Feng D. Recent progress in the role of autophagy in neurological diseases. Cell Stress. 2019 Apr 29;3(5):141-161. doi: 10.15698/cst2019.05.186. PMID: 31225510; PMCID: PMC6551859.
https://pubmed.ncbi.nlm.nih.gov/31225510/
96. Aman Y, Schmauck-Medina T, Hansen M, Morimoto RI, Simon AK, Bjedov I, Palikaras K, Simonsen A, Johansen T, Tavernarakis N, Rubinsztein DC, Partridge L, Kroemer G, Labbadia J, Fang EF. Autophagy in healthy aging and disease. Nat Aging. 2021 Aug;1(8):634-650. doi: 10.1038/s43587-021-00098-4. Epub 2021 Aug 12. PMID: 34901876; PMCID: PMC8659158.
https://pubmed.ncbi.nlm.nih.gov/34901876/
97. Zhang LX, Li CX, Kakar MU, Khan MS, Wu PF, Amir RM, Dai DF, Naveed M, Li QY, Saeed M, Shen JQ, Rajput SA, Li JH. Resveratrol (RV): A pharmacological review and call for further research. Biomed Pharmacother. 2021 Nov;143:112164. doi: 10.1016/j.biopha.2021.112164. Epub 2021 Oct 2. PMID: 34649335.
https://pubmed.ncbi.nlm.nih.gov/34649335/
98. Tejada-Vera B. Mortality from Alzheimer's disease in the United States: data for 2000 and 2010. NCHS Data Brief. 2013 Mar;(116):1-8. PMID: 23742787.
https://pubmed.ncbi.nlm.nih.gov/23742787/
99. Tasker RA, Adams-Marriott AL, Shaw CA. New animal models of progressive neurodegeneration: tools for identifying targets in predictive diagnostics and presymptomatic treatment. EPMA J. 2010 Jun;1(2):217-27. doi: 10.1007/s13167-010-0019-0. Epub 2010 Jun 9. PMID: 23199060; PMCID: PMC3405326.
https://pubmed.ncbi.nlm.nih.gov/23199060/
100. Pierzynowska K, Gaffke L, Cyske Z, Puchalski M, Rintz E, Bartkowski M, Osiadły M, Pierzynowski M, Mantej J, Piotrowska E, Węgrzyn G. Autophagy stimulation as a promising approach in treatment of neurodegenerative diseases. Metab Brain Dis. 2018 Aug;33(4):989-1008. doi: 10.1007/s11011-018-0214-6. Epub 2018 Mar 14. PMID: 29542037; PMCID: PMC6060747.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6060747/
101. Sose PM, Doshi GM, Kale PP. An Update on Autophagy as a Target in the Treatment of Alzheimer's Disease. Curr Drug Targets. 2023;24(7):547-567. doi: 10.2174/1389450124666230417104325. PMID: 37070441.
https://pubmed.ncbi.nlm.nih.gov/37070441/
102. Singh S, Bhatt LK. Targeting cellular senescence: a potential therapeutic approach for Alzheimer's disease. Curr Mol Pharmacol. 2023 Jun 1. doi: 10.2174/1874467217666230601113430. Epub ahead of print. PMID: 37264659.
https://pubmed.ncbi.nlm.nih.gov/37264659/
103. Obrador E, Salvador-Palmer R, López-Blanch R, Dellinger RW, Estrela JM. NAD+ Precursors and Antioxidants for the Treatment of Amyotrophic Lateral Sclerosis. Biomedicines. 2021 Aug 12;9(8):1000. doi: 10.3390/biomedicines9081000. PMID: 34440204; PMCID: PMC8394119
https://pubmed.ncbi.nlm.nih.gov/34440204/
Senescent cells reentering the cell cycle: senescence escape
104. Zeng S, Shen WH, Liu L. Senescence and Cancer. Cancer Transl Med. 2018 May-Jun;4(3):70-74. doi: 10.4103/ctm.ctm_22_18. Epub 2018 Jun 29. PMID: 30766922; PMCID: PMC6372122.
https://pubmed.ncbi.nlm.nih.gov/30766922/
105. Roger L, Tomas F, Gire V. Mechanisms and Regulation of Cellular Senescence. Int J Mol Sci. 2021 Dec 6;22(23):13173. doi: 10.3390/ijms222313173. PMID: 34884978; PMCID: PMC8658264.
https://pubmed.ncbi.nlm.nih.gov/34884978/
106. Zampetidis CP, Papantonis A, Gorgoulis VG. Escape from senescence: revisiting cancer therapeutic strategies. Mol Cell Oncol. 2022 Feb 15;9(1):2030158. doi: 10.1080/23723556.2022.2030158. PMID: 35252554; PMCID: PMC8890391.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8890391/