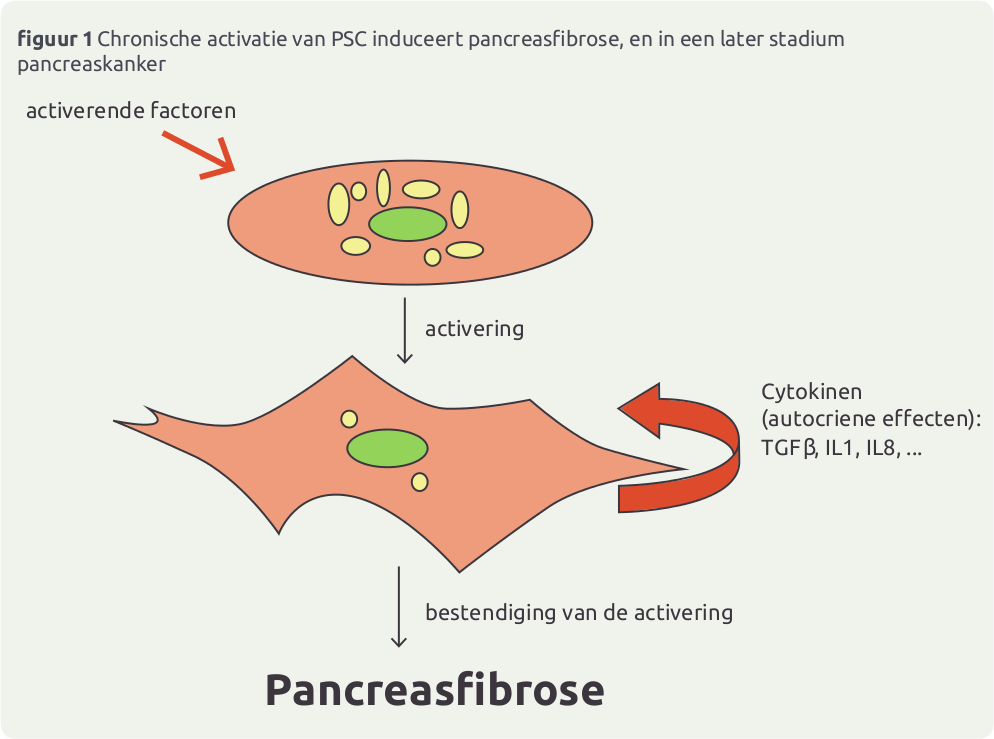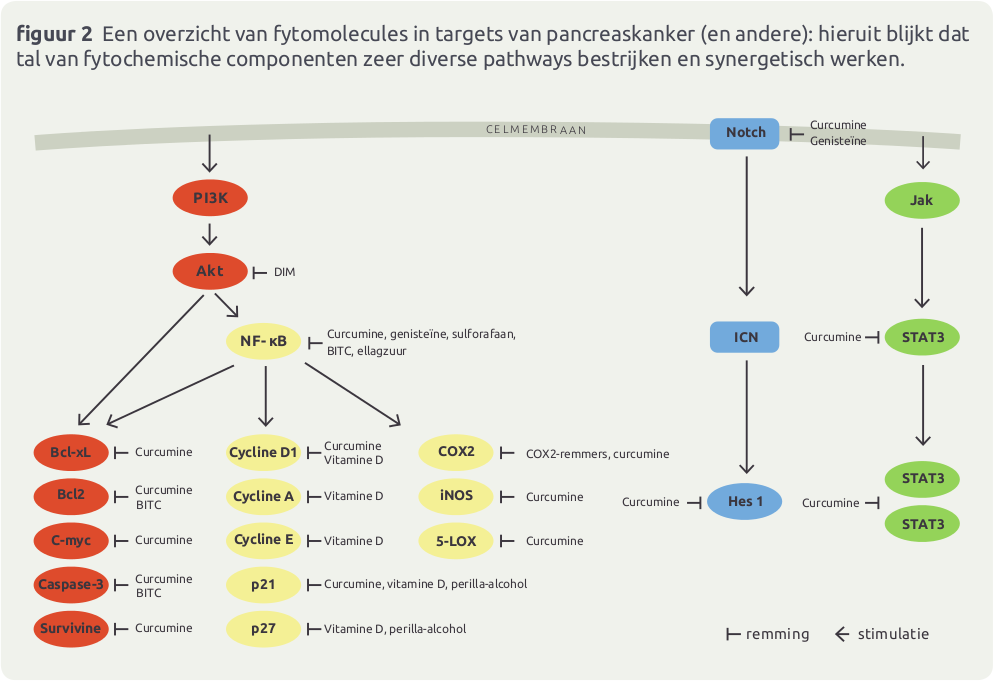
Nederland zit in de top 5 voor wat betreft kankercijfers en mortaliteit, met 40.000 kankerdoden per jaar. Ook pancreaskanker scoort hoog en gaat in stijgende lijn: van 1750 in 2006, naar 2481 in 2010 …
België blinkt uit door totale afwezigheid van cijfers.
Beste bezoeker, u heeft geen toegang.
Enkel (web)abonnees hebben toegang tot tijdschriftartikelen. Het webabonnement is nog in de maak.
U kunt zich wel alvast (gratis) registreren en tal van andere webartikelen raadplegen!
Auteur
Verschenen in
Referenties
Lijst carcinogenen: WHO publications: chemical toxicology, carcinogenicityhttp://www.who.int/dsa/cat98/chemtox8.htm
Andersen DK et al. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013 Nov;42(8):1227-37.
Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007 Jul;246(1):52-60.
Nys M. Spijsverteringsenzymen, miskend en onderschat, maar essentieel voor gezondheid en leven. AT&A 2012; 5:18-23.
Pruimboom L. De lever/pancreas/resistenties binnen de klinische PNI. 2e jaar kPNI. 2011.
Feinberg AP, Tycko B: The history of cancer epigenetics. Nat Rev Cancer 2004, 4(2):143-153.
McCleary-Wheeler AL et al. Insights into the epigenetic mechanisms controlling pancreatic carcinogenesis. Cancer Lett. 2013 Jan 28;328(2):212-21.
Longnecker DS. Abnormal methyl metabolism in pancreatic toxicity and diabetes. J Nutr. 2002 Aug;132(8 Suppl):2373S-2376S
Gao J, Li Z, Chen Z, Shao J, Zhang L, Xu G, Tu Z, Gong Y: Antisense Smo under the control of the PTCH1 promoter delivered by an adenoviral vector inhibits the growth of human pancreatic cancer. Gene Ther 2006, 13(22):1587-1594.
Wang W, Gao J, Man XH, Li ZS, Gong YF: Significance of DNA methyltransferase-1 and histone deacetylase-1 in pancreatic cancer. Oncol Rep 2009, 21(6):1439-1447.
Ushijima T: Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer 2005, 5(3):223-231.
Brune K, Hong SM, Li A, Yachida S, Abe T, Griffith M, Yang D, Omura N, Eshleman J, Canto M, Schulick R, Klein AP, Hruban RH, Iacobuzio-Donohue C, Goggins M: Genetic and epigenetic alterations of familial pancreatic cancers. Cancer Epidemiol Biomarkers Prev 2008, 17(12):3536-3542.
Bradshaw AD, Sage EH: SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 2001, 107(9):1049-1054.
Brekken RA, Sage EH: SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 2001, 19(8):816-827.
Jendraschak E, Sage EH: Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol 1996, 7(3):139-146.
Yan Q, Sage EH: SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 1999, 47(12):1495-1506.
Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M: SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene 2003, 22(32):5021-5030.
Lowenfels AB, Maisonneuve P: Risk factors for pancreatic cancer. J Cell Biochem 2005, 95(4):649-656.
Hanoun N et al. The silencing of microRNA 148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin Chem. 2010 Jul;56(7):1107-18.
Biewusch K, Heyne M, Grützmann R, Pilarsky C. DNA methylation in pancreatic cancer: protocols for the isolation of DNA and bisulfite modification. Methods Mol Biol. 2012;863:273-80.
Schernhammer E. Plasma folate, vitamins B6, B12, and homocysteine and pancreatic cancer risk in four large cohorts. Cancer Research 2007; 67(11)
Hernández-Muñoz I, Skoudy A, Real FX, Navarro P. Pancreatic ductal adenocarcinoma: cellular origin, signaling pathways and stroma contribution. Pancreatology. 2008;8(4-5):462-9.
Hamada S, Masamune A, Shimosegawa T. Inflammation and pancreatic cancer: disease promoter and new therapeutic target. J Gastroenterol. 2013 Nov 30. [Epub ahead of print]
Erkan M et al. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med. 2012 Mar;12(3):288-303.
Luo G et al. Stroma and pancreatic ductal adenocarcinoma: an interaction loop. Biochim Biophys Acta. 2012 Aug;1826(1):170-8.
Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012 Mar;27 Suppl 2:69-74.
Eguchi D et al. Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor. J Surg Res. 2013 May;181(2):225-33.
Feig C et al. The pancreas cancer microenvironment. Clin Cancer Res. 2012 Aug 15;18(16):4266-76.
Subramaniam D, Ramalingam S, Houchen CW, Anant S. Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev Med Chem. 2010;10:359–71.
Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer. 2008 Jul 24;7:64.
Masamune A et al. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G99-G108.
Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012 Aug 28;3:344.
Ceyhan GO et al. Pancreatic neuropathy results in "neural remodeling" and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am J Gastroenterol. 2009 Oct;104(10):2555-65.
Demir IE et al. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010 Jul 14;2(3):1513-27.
Wang K et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis. 2013 Oct 26. [Epub ahead of print]
Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012 Aug;1826(1):112-20.
Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012 Apr 17;3:97.
Omary MB, Pandol SJ et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007; 117(1):50-9.
Dinga Z et al. Glial fibrillary acidic protein promoter targets pancreatic stellate cells. http://www.sciencedirect.com Liver, Pancreas and Biliary Tract March 2009; 41(3): 229–236.
Mace TA et al. Pancreatic cancer associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res Published Online 2013 March 20; doi: 10.1158/0008-5472.CAN-12-4601
Apte MV et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004 Oct;29(3):179-87.
Vonlaufen A et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008 Apr 1;68(7):2085-93.
Bachem MG et al. Pancreatic stellate cells--role in pancreas cancer. Langenbecks Arch Surg. 2008 Nov;393(6):891-900.
Zhang W et al. Expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in pancreatic neoplasm and pancreatic stellate cells. Cancer Biol Ther. 2007 Feb;6(2):218-27.
Schneiderhan W et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci. 2007 Feb 1;120(Pt 3):512-9.
Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008 Oct;149(2):319-28.
Łukaszewicz M et al. The role of metalloproteinases and their inhibitors in pancreatic cancer]. Postepy Hig Med Dosw (Online). 2008 Apr 7;62:141-7.
Bister V et al. Increased expression of matrix metalloproteinases-21 and -26 and TIMP-4 in pancreatic adenocarcinoma. Mod Pathol. 2007 Nov;20(11):1128-40.
Yamamoto H et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol. 2001 Feb 15;19(4):1118-27.
Jia YJ et al. Rat pancreatic stellate-like cells differentiate into neuron. Endocrine Abstracts 2004; 8:26.
Erkan M et al. Current consensus and discussion on pancreatic stellate cell research. 2012; gut.bmj.com/content/61/2/172.full.pdf
Scarlett CJ et al. Recruitment and Activation of Pancreatic Stellate Cells from the Bone Marrow in Pancreatic Cancer: A Model of Tumor-Host Interaction. PLOS one 2011, Oct 14 DOI: 10.1371.
Masamune A et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009 Nov;7(11 Suppl):S48-54.
Hamada S, Masamune A, Shimosegawa T. Alteration of pancreatic cancer cell functions by tumor-stromal cell interaction. Front Physiol. 2013 Nov 1;4:318.
Carbone C, Melisi D. NF-κB as a target for pancreatic cancer therapy. Expert Opin Ther Targets. 2012 Apr;16 Suppl 2:S1-10.
Zhang Z, Rigas B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review). Int J Oncol. 2006 Jul;29(1):185-92.
Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J Gastroenterol Hepatol. 2006 May;21(5):850-8.
Zhang Y, Dang C, Ma Q, Shimahara Y. Expression of nerve growth factor receptors and their prognostic value in human pancreatic cancer. Oncol Rep. 2005 Jul;14(1):161-71.
Kayahara M, Nakagawara H, Kitagawa H, Ohta T. The nature of neural invasion by pancreatic cancer. Pancreas. 2007 Oct;35(3):218-23.
Demir IE et al. Neural invasion in pancreatic cancer: the past, present and future. Cancers (Basel). 2010 Jul 14;2(3):1513-27.
Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012 Aug;1826(1):112-20.
Gil Z et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010 Jan 20;102(2):107-18.
Cavel O et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012 Nov 15;72(22):5733-43.
Bapat AA et al. Perineural invasion and associated pain in pancreatic cancer. Nature Reviews Cancer 2011; 11, 695-707
Liu H et al. Therapeutic Potential of Perineural Invasion, Hypoxia and Desmoplasia in Pancreatic. Cancer Curr Pharm Des. 2012; 18(17): 2395–2403.
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009 Aug 1;115(15):3379-91.
Hamada S, Masamune A, Shimosegawa T. Novel therapeutic strategies targeting tumor-stromal interactions in pancreatic cancer. Front Physiol. 2013; 4: 331.
Demir IE et al. Neural Invasion in Pancreatic Cancer: The Past, Present and Future. Review. Cancers 2010, 2, 1513-1527.
Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol. 2006, 21, 850–858.
Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. Muc1 is a counter-receptor for myelin-associated glycoprotein (siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007, 67, 10222–10229.
Kolb A et al. Glucagon/insulin ratio as a potential biomarker for pancreatic cancer in patients with new-onset diabetes mellitus. Cancer Biol Ther. 2009 Aug;8(16):1527-33.
Zhu ZW et al. Nerve growth factor exerts differential effects on the growth of human pancreatic cancer cells. Clin Cancer Res. 2001 Jan;7(1):105-12.
Moriyama T, Ohuchida K, Mizumoto K, Cui L, Ikenaga N, Sato N, Tanaka M. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–68.
Jung DE, Wen J, Oh T, Song SY. Differentially expressed micro-RNAs in pancreatic cancer stem cells. Pancreas. 2011;40:1180–7.
De La OJ, Murtaugh LC. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860–4.
Wang Z, Sengupta R, Banerjee S, Li Y, Zhang Y, Rahman KM, Aboukameel A, Mohammad R, Majumdar AP, Abbruzzese JL, Sarkar FH. Epidermal growth factor receptor-related protein inhibits cell growth and invasion in pancreatic cancer. Cancer Res. 2006;66:7653–60.
Martelli AM, Evangelisti C, Follo MY, Ramazzotti G, Fini M, Giardino R, Manzoli L, McCubrey JA, Cocco L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in cancer stem cells. Curr Med Chem. 2011;18:2715–26.
Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41.
Ahmad S, Chowdhury TA, Boucher BJ. Diabetes and cancer: Could vitamin D provide the link? J Diabetes Complications. 2013 Mar-Apr;27(2):184-90.
Muniraj T, Chari ST. Diabetes and pancreatic cancer. Minerva Gastroenterol Dietol. 2012 Dec;58(4):331-45.
Andersen DK. Diabetes and cancer: placing the association in perspective. Curr Opin Endocrinol Diabetes Obes. 2013 Apr;20(2):81-6.
Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012 Jan;51(1):64-74.
Holick MF. Vitamin D, sunlight and cancer connection. Anticancer Agents Med Chem. 2013 Jan;13(1):70-82.
Tran B, Whiteman DC et al. Association between ultraviolet radiation, skin sun sensitivity and risk of pancreatic cancer. Cancer Epidemiol. 2013 Dec;37(6):886-92.
Cho M, Peddi PF et al. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J Transl Med. 2013 Sep 8;11(1):206.
Anderson LN et al. Genetic variants in vitamin d pathway genes and risk of pancreas cancer; results from a population-based case-control study in ontario, Canada. PLoS One. 2013 Jun 24;8(6):e66768.
Lashinger LM, Ford NA, Hursting SD. Interacting Inflammatory and Growth Factor Signals Underlie the Obesity–Cancer Link. J. Nutr. February 1, 2014 jn.113.178533
Kanemaru M, Maehara N, Chijiiwa K. Antiproliferative effect of 1α,25-dihydroxyvitamin D3 involves upregulation of cyclin-dependent kinase inhibitor p21 in human pancreatic cancer cells. Hepatogastroenterology. 2013 Jul-Aug;60(125):1199-205.
Li L et al. Association of vitamin D receptor gene polymorphisms with pancreatic cancer: A pilot study in a North China Population. Oncol Lett. 2013 May;5(5):1731-1735.
Kim YS, Farrar W, Colburn NH, Milner JA. Cancer stem cells: potential target for bioactive food components. J Nutr Biochem. 2012 Jul;23(7):691-8.
Li Y, Wicha MS, Schwartz SJ, Sun D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem. 2011 Sep;22(9):799-806.
Dandawate P, Padhye S, Ahmad A, Sarkar FH. Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv Transl Res. 2013 Apr 1;3(2):165-182.
Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2010 Jun;7(6):347-56.
Jansen RJ et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. J Gastrointest Cancer. 2013 Jun;44(2):152-61.
Banim PJ et al. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013 Oct;62(10):1489-96.
van den Brandt PA, Goldbohm RA. Nutrition in the prevention of gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2006;20(3):589-603.
Woutersen RA, Appel MJ, Van Garderen-Hoetmer A. Modulation of pancreatic carcinogenesis by antioxidants. Food Chem Toxicol. 1999 Sep-Oct;37(9-10):981-4.
Levin B. An overview of preventive strategies for pancreatic cancer. Ann Oncol. 1999;10 Suppl 4:193-6.
Stolzenberg-Solomon RZ et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol. 2002 May 1;155(9):783-92.
Bobe G et al. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland). Cancer Epidemiol Biomarkers Prev. 2008 Mar;17(3):553-62.
Han X et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) Study. Cancer. 2013 Apr 1;119(7):1314-20.
Banim PJ, Luben R, McTaggart A, Welch A, Wareham N, Khaw KT, Hart AR. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013 Oct;62(10):1489-96.
Amaral AF et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012 Nov;61(11):1583-8.
Heinen MM, Verhage BA, Goldbohm RA, van den Brandt PA. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. Int J Cancer. 2012 Jan 1;130(1):147-58.
Gupta S et al. Molecular determinants of retinoic acid sensitivity in pancreatic cancer. Clin Cancer Res. 2012 Jan 1;18(1):280-9.
Froeling FE et al.Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology. 2011 Oct;141(4):1486-97, 1497.e1-14.
Appel MJ, Woutersen RA. Effects of dietary beta-carotene and selenium on initiation and promotion of pancreatic carcinogenesis in azaserine-treated rats. Carcinogenesis. 1996 Jul;17(7):1411-6.
Stolzenberg-Solomon RZ et al. Vitamin E intake, alpha-tocopherol status, and pancreatic cancer in a cohort of male smokers. Am J Clin Nutr. 2009 Feb;89(2):584-91.
Jackson JA, Riordan HD, Hunninghake RE, Riordan NH: High-dose intravenous vitamin C and long-term survival of a patient with cancer of the head of the pancreas. ] Orthomol Med 1995; 10: 87-88.
Jackson AJ et al. A Child with Metastatic Sarcoma and A Patient with Cancer of the Head of the Pancreas. JOM 2008; 23(1):41-2.
Bao Y et al. Folate intake and risk of pancreatic cancer: pooled analysis of prospective cohort studies. J Natl Cancer Inst. 2011 Dec 21;103(24):1840-50.
Larsson SC, Håkansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006 Mar 15;98(6):407-13.
Larsson SC, Giovannucci E, Wolk A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology. 2007 Jan;132(1):113-8.
Stolzenberg-Solomon RZ et al. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol. 2001 Apr 1;153(7):680-7.
Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006 Oct;131(4):1271-83.
Han X et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) Study. Cancer. 2013 Apr 1;119(7):1314-20.
Sanmartín C, Plano D, Font M, Palop JA. Kinase regulation by sulfur and selenium containing compounds. Curr Cancer Drug Targets. 2011 May;11(4):496-523.
El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds.
Aichler M et al. Selenium status alters tumour differentiation but not incidence or latency of pancreatic adenocarcinomas in Ela-TGF-alpha p53+/ mice. Carcinogenesis. 2007 Sep;28(9):2002-7.
Banim PJ, Luben R, McTaggart A, Welch A, Wareham N, Khaw KT, Hart AR. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013 Oct;62(10):1489-96.
Amaral AF et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012 Nov;61(11):1583-8.
Nys M. Selenium: een fysiologische duizendpoot Deel 3. Het essentiële gif. A&A 2005; 2.
Vergote G et al. Selenium, van kleurstof tot chemopreventief middel. OF 2004; 50:249-252.
M. Estrelaa JM, Ortegaa A, Obradora E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sc2006; 43(2):143 - 181
Yang et al. L-cysteine administration attenuates pancreatic fibrosis induced by TNBS in rats by inhibiting the activation of pancreatic stellate cell. PLoS One. 2012;7(2):e31807.
Johnson JL, de Mejia EG. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol Nutr Food Res. 2013 Dec;57(12):2112-27.
Johnson JL et al. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol 2013; Oct 60:83-91.
Johnson JL, de Mejia EG. Interactions between dietary flavonoids apigenin or luteolin and chemotherapeutic drugs to potentiate anti-proliferative effect on human pancreatic cancer cells, in vitro. Food Chem Toxicol. 2013 Oct;60:83-91.
Lan X et al. Effects of garlic oil on pancreatic cancer cells. Asian Pac J Cancer Prev. 2013;14(10):5905-10.
Z Tsang SW et al. Rhein, a natural anthraquinone derivative, attenuates the activation of pancreatic stellate cells and ameliorates pancreatic fibrosis in mice with experimental chronic pancreatitis. PLoS One. 2013 Dec 3;8(12):e82201.
Yang et al. L-cysteine administration attenuates pancreatic fibrosis induced by TNBS in rats by inhibiting the activation of pancreatic stellate cell. PLoS One. 2012;7(2):e31807.
Zhu WH et al. Strategies of functional food for cancer prevention in human beings. Asian Pac J Cancer Prev. 2013;14(3):1585-92.
Rosa FT, Zulet MÁ, Marchini JS, Martínez JA. Bioactive compounds with effects on inflammation markers in humans. Int J Food Sci Nutr. 2012 Sep;63(6):749-65.
Amaral AF et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012 Nov;61(11):1583-8.
Johnson JL, de Mejia EG. Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK-3β/NF-κB signaling cascade. Mol Nutr Food Res. 2013 Dec;57(12):2112-27.
Amalendu P Ranjan, Anindita Mukerjee, Lawrence Helson, Rohan Gupta, Jamboor K Vishwanatha. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: inhibition of tumor growth and angiogenesis. Anticancer Res. 2013 Sep ;33(9):3603-9.
Rai B et al. Curcumin exhibits anti-pre-cancer activity by increasing levels of vitamins C and E, and preventing lipid peroxidation and DNA damage. J Oral Sci. 2010;52(2):251-6.
Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007 Aug 1;43(3):444-53.
Zhou Y, Zheng S, Lin J, Zhang QJ, Chen A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab Invest. 2007 May;87(5):488-98.
Masamune A et al. Curcumin blocks activation of pancreatic stellate cells. J Cell Biochem. 2006 Apr 1;97(5):1080-93.
Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011 Jan 1;3:515-28.
Kunnumakkara AB et al. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007 Apr 15;67(8):3853-61.
Ali S et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010 May 1;70(9):3606-17.
Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13.
Bao B, Sarkar FH et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7(12):e50165.
Padhye S, Chavan D, Pandey S, Deshpande J, Swamy KV, Sarkar FH. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev Med Chem. 2010;10:372–87.
Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850.
Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010;70:3606–17.
Bao B et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012 Jan 1;72(1):335-45.
Dandawate PR et al. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm Res. 2012 Jul;29(7):1775-86.
Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–45.
Thakkar A, Sutaria D, Grandhi BK, Wang J, Prabhu S. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncol Rep. 2013 Apr;29(4):1671-7.
Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2013 Jan;373(1-2):217-27.
Li Y et al. Sulforaphane inhibits pancreatic cancer through disrupting Hsp90-p50(Cdc37) complex and direct interactions with amino acids residues of Hsp90. J Nutr Biochem. 2012 Dec;23(12):1617-26.
Boreddy SR, Sahu RP, Srivastava SK. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3. PLoS One. 2011;6(10):e25799.
Hutzen B et al. Dietary agent, benzyl isothiocyanate inhibits signal transducer and activator of transcription 3 phosphorylation and collaborates with sulforaphane in the growth suppression of PANC-1 cancer cells. Cancer Cell Int. 2009 Aug 27;9:24.
Basu A, Haldar S. Anti-proliferative and proapoptotic effects of benzyl isothiocyanate on human pancreatic cancer cells is linked to death receptor activation and RasGAP/Rac1 down-modulation. Int J Oncol. 2009 Sep;35(3):593-9.
Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009 Oct;30(10):1744-53.
Kuroiwa Y, Nishikawa A, Kitamura Y, Kanki K, Ishii Y, Umemura T, Hirose M. Protective effects of benzyl isothiocyanate and sulforaphane but not resveratrol against initiation of pancreatic carcinogenesis in hamsters. Cancer Lett. 2006;241:275–80.
Zanichelli F, Capasso S, Cipollaro M, Pagnotta E, Carteni M, Casale F, Iori R, Galderisi U. Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr) 2012;34:281–93.
Li Y, Zhang T, Schwartz SJ, Sun D. Sulforaphane potentiates the efficacy of 17-allylamino 17-demethoxygeldanamycin against pancreatic cancer through enhanced abrogation of Hsp90 chaperone function. Nutr Cancer. 2011;63(7):1151-9.
Ohara M et al. Benzyl isothiocyanate sensitizes human pancreatic cancer cells to radiation by inducing apoptosis. Int J Mol Med. 2011 Dec;28(6):1043-7.
Basu A et al. MicroRNA-375 and MicroRNA-221: Potential Noncoding RNAs Associated with Antiproliferative Activity of Benzyl Isothiocyanate in Pancreatic Cancer. Genes Cancer. 2011 Feb;2(2):108-19.
Naumann P et al. Autophagy and cell death signaling following dietary sulforaphane act independently of each other and require oxidative stress in pancreatic cancer. Int J Oncol. 2011 Jul;39(1):101-9.
Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin Cancer Res. 2011 Apr 1;17(7):1784-95.
Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004 Sep;25(9):1701-9.
Batra S, Sahu RP, Kandala PK, Srivastava SK. Benzyl isothiocyanate-mediated inhibit ion of histone deacetylase leads to NF-kappaB turnoff in human pancreatic carcinoma cells. Mol Cancer Ther. 2010 Jun;9(6):1596-608.
Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009 Feb 4;101(3):176-93.
Basu A, Haldar S. Dietary isothiocyanate mediated apoptosis of human cancer cells is associated with Bcl-xL phosphorylation. Int J Oncol. 2008 Oct;33(4):657-63.
Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, Buchler MW, Salnikov AV, Herr I. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19:188–95.
de Figueiredo SM et al. The anti-oxidant properties of isothiocyanates: a review. Recent Pat Endocr Metab Immune Drug Discov. 2013 Sep;7(3):213-25.
Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012 Oct;33(10):1833-42.
Rausch V et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010 Jun 15;70(12):5004-13.
Kallifatidis G et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011 Jan;19(1):188-95.
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemo-resistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33.
Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed) 2011;3:515–28.
Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011 Jan 1;3:515-28.
Zhou W et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010 Sep;37(3):551-61.
McMillan B, Riggs DR, Jackson BJ, Cunningham C, McFadden DW. Dietary influence on pancreatic cancer growth by catechin and inositol hexaphosphate. J Surg Res. 2007 Jul;141(1):115-9.
Shankar S, Marsh L, Srivastava RK. EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Mol Cell Biochem. 2013 Jan;372(1-2):83-94.
Asaumi H et al. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits ethanol-induced activation of pancreatic stellate cells. Eur J Clin Invest. 2006 Feb;36(2):113-22.
Chen A, Zhang L, Xu J, Tang J. The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J. 2002 Dec 15;368(Pt 3):695-704.
Yumei F, Zhou Y, Zheng S, Chen A. The antifibrogenic effect of (-)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells. Lab Invest. 2006 Jul;86(7):697-709.
Fu Y, Chen A. The phyto-chemical (-)-epigallocatechin gallate suppresses gene expression of epidermal growth factor receptor in rat hepatic stellate cells in vitro by reducing the activity of Egr-1. Biochem Pharmacol. 2006 Jul 14;72(2):227-38.
Suzuki N et al. Ellagic acid inhibits pancreatic fibrosis in male Wistar Bonn/Kobori rats. Dig Dis Sci. 2009 Apr;54(4):802-10.
Masamune A et al. Ellagic acid blocks activation of pancreatic stellate cells. Biochem Pharmacol. 2005 Sep 15;70(6):869-78.
Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One. 2011;6(9):e25166.
Zhou JH et al. Resveratrol induces apoptosis in pancreatic cancer cells. Chin Med J (Engl). 2011 Jun;124(11):1695-9.
Shamim U et al. Resveratrol-induced apoptosis is enhanced in low pH environments associated with cancer. J Cell Physiol. 2012 Apr;227(4):1493-500.
Neves AR, Lucio M, Lima JL, Reis S. Resveratrol in medicinal chemistry: a critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr Med Chem. 2012;19:1663–81.
Cui J et al. Antiproliferative effect of resveratrol in pancreatic cancer cells. Phytother Res. 2010 Nov;24(11):1637-44.
Shankar S et al. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic m PLoS One. 2011 Jan 31;6(1):e16530.
Cho IR et al. SIRT1 inhibits proliferation of pancreatic cancer cells expressing pancreatic adenocarcinoma up-regulated factor (PAUF), a novel oncogene, by suppression of β-catenin. Biochem Biophys Res Commun. 2012 Jun 29;423(2):270-5.
Mo W et al. Resveratrol inhibits proliferation and induces apoptosis through the hedgehog signaling pathway in pancreatic cancer cell. Pancreatology. 2011;11(6):601-9.
Bernhaus A et al. Antitumor effects of KITC, a new resveratrol derivative, in AsPC-1 and BxPC-3 human pancreatic carcinoma cells. Invest New Drugs. 2009 Oct;27(5):393-401.
Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011;130:387–98.
Nambiar D et al. In vitro and in vivo anticancer efficacy of silibinin against human pancreatic cancer BxPC-3 and PANC-1 cells. Proc Amer Assoc Cancer Res 2005; 46
Bae GS et al. The inhibitory effects of Nardostachys jatamansi on alcoholic chronic pancreatitis. BMB Rep. 2012 Jul;45(7):402-7.
Kaur M, Deep G, Jain AK, Raina K, Agarwal C, Wempe MF, Agarwal R. Bitter melon juice activates cellular energy sensor AMP-activated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis. 2013 Jul;34(7):1585-92.
Kwatra D, Venugopal A, Standing D, Ponnurangam S, Dhar A, Mitra A, Anant S. Bitter melon extracts enhance the activity of chemotherapeutic agents through the modulation of multiple drug resistance. J Pharm Sci. 2013 Dec;102(12):4444-54.
Manoharan G, Jaiswal SR, Singh J. Effect of α, β momorcharin on viability, caspase activity, cytochrome c release and on cytosolic calcium levels in different cancer cell lines. Mol Cell Biochem. 2013 Dec 3. [Epub ahead of print]
Waiyaput W, Payungporn S, Issara-Amphorn J, Panjaworayan NT. Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells. BMC Complement Altern Med. 2012 Dec 6;12:246. doi: 10.1186/1472-6882-12-246.
Weng JR, Bai LY, Chiu CF, Hu JL, Chiu SJ, Wu CY. Cucurbitane Triterpenoid from Momordica charantia Induces Apoptosis and Autophagy in Breast Cancer Cells, in Part, through Peroxisome Proliferator-Activated Receptor γ Activation. Evid Based Complement Alternat Med. 2013;2013:935675.
Thoennissen NH et al. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009 Jul 15;69(14):5876-84.
Li CJ, Tsang SF, Tsai CH, Tsai HY, Chyuan JH, Hsu HY. Momordica charantia Extract Induces Apoptosis in Human Cancer Cells through Caspase- and Mitochondria-Dependent Pathways. Evid Based Complement Alternat Med. 2012;2012:261971.
Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH, Ng TB. The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer Lett. 2012 Nov 1;324(1):66-74.
Fang EF, Zhang CZ, Zhang L, Fong WP, Ng TB. In vitro and in vivo anticarcinogenic effects of RNase MC2, a ribonuclease isolated from dietary bitter gourd, toward human liver cancer cells. Int J Biochem Cell Biol. 2012 Aug;44(8):1351-60.
Wang X, Sun W, Cao J, Qu H, Bi X, Zhao Y. Structures of new triterpenoids and cytotoxicity activities of the isolated major compounds from the fruit of Momordica charantia L. J Agric Food Chem. 2012 Apr 18;60(15):3927-33.
Zhang J, Huang Y, Kikuchi T, Tokuda H, Suzuki N, Inafuku K, Miura M, Motohashi S, Suzuki T, Akihisa T. Cucurbitane triterpenoids from the leaves of Momordica charantia, and their cancer chemopreventive effects and cytotoxicities.
Brennan VC, Wang CM, Yang WH. Bitter melon (Momordica charantia) extract suppresses adrenocortical cancer cell proliferation through modulation of the apoptotic pathway, steroidogenesis, and insulin-like growth factor type 1 receptor/RAC-α serine/threonine-protein kinase signaling. J Med Food. 2012 Apr;15(4):325-34.
Xia J et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets. 2012 Dec;13(14):1750-6.
Han L et al. The effects of genistein on transforming growth factor-β1-induced invasion and metastasis in human pancreatic cancer cell line Panc-1 in vitro. Chin Med J (Engl). 2012 Jun;125(11):2032-40.
Wang Z et al. FoxM1 is a novel target of a natural agent in pancreatic cancer. Pharm Res. 2010 Jun;27(6):1159-68.
Bao B et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011 Sep;112(9):2296-306.
Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int J Cancer. 2006 Apr 15;118(8):1930-6.
Bao B et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011 Aug 1;307(1):26-36. Cheng S, Eliaz I, Lin J, Sliva D.
Shimizu T et al. Holy Basil leaf extract decreases tumorigenicity and metastasis of aggressive human pancreatic cancer cells in vitro and in vivo: potential role in therapy. Cancer Lett. 2013 Aug 19;336(2):270-80.
Bhattacharyya P, Bishayee A. Ocimum sanctum Linn. (Tulsi): an ethnomedicinal plant for the prevention and treatment of cancer. Anticancer Drugs. 2013 Aug;24(7):659-66.
Baliga MS et al. Ocimum sanctum L (Holy Basil or Tulsi) and its phytochemicals in the prevention and treatment of cancer. Nutr Cancer. 2013;65 Suppl 1:26-35.
Rosendahl AH, Sun C, Wu D, Andersson R. Polysaccharide-K (PSK) increases p21(WAF/Cip1) and promotes apoptosis in pancreatic cancer cells. Pancreatology. 2012 Nov-Dec;12(6):467-74.
Sun C et al. Polysaccharide-K (PSK) in cancer--old story, new possibilities? Curr Med Chem. 2012;19(5):757-62.
Triterpenes from Poria cocos suppress growth and invasiveness of pancreatic cancer cells through the downregulation of MMP-7. Int J Oncol. 2013 Jun;42(6):1869-74. Ríos JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011 May;77(7):681-91.
Feng YL et al. Chemical constituents of surface layer of Poria cocos and their pharmacological properties (I). Zhongguo Zhong Yao Za Zhi. 2013 Apr;38(7):1098-102.
Akihisa T et al. Anti-tumor-promoting effects of 25-methoxyporicoic acid A and other triterpene acids from Poria cocos. J Nat Prod. 2009 Oct;72(10):1786-92.
Shan H et al. Reversal of multidrug resistance of KBV200 cells by triterpenoids isolated from Poria cocos. Planta Med. 2012 Mar;78(5):428-33.
Suenaga S et al. Active Hexose-correlated Compound Down-regulates HSP27 of Pancreatic Cancer Cells, and Helps the Cytotoxic Effect of Gemcitabine. Anticancer Res. 2014 Jan;34(1):141-6.
Jiang M et al. Influence of betulinic acid on proliferation, migration, cell cycle and apoptosis of pancreatic cancer cells. Zhongguo Zhong Yao Za Zhi. 2010 Nov;35(22):3056-9.
Taji S, Yamada T, Wada S, Tokuda H, Sakuma K, Tanaka R. Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem 2008; 43 (11): 2373–9.
Nomura M, Takahashi T, Uesugi A, Tanaka R, Kobayashi S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res 2008; 28 (5A): 2691–6
Mullauer FB, Kessler JH, Medema JP. Betulin is a potent anti-tumor agent that is enhanced by cholesterol. PLoS ONE 2009; 4 (4): e1
Yu CC et al. Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J Nutr Biochem. 2012 Aug;23(8):900-7.
Chiang PC et al. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: a crucial role of AMPK and mTOR pathways. Biochem Pharmacol. 2010 Jan 15;79(2):162-71.
Ginsenoside Rh2 inhibits proliferation, migration and invasion and induces apoptosis of the human pancreatic cancer cell line Bxpc-3.
Tang XP et al. Effects of ginsenoside Rh2 on growth and migration of pancreatic cancer cells. World J Gastroenterol. 2013 Mar 14;19(10):1582-92.
Li Y et al. Effect of Jinlong capsule on proliferation and apoptosis of human pancreatic cancer cells BxPC-3. J Tradit Chin Med. 2013 Apr;33(2):205-10.
Banerjee S et al. Triptolide-induced Cell Death in Pancreatic Cancer Is Mediated by O-GlcNAc Modification of Transcription Factor Sp1. November 22, 2013 The Journal of Biological Chemistry, 288, 33927-33938.
Phillips PA et al. Triptolide Induces Pancreatic Cancer Cell Death via Inhibition of Heat Shock Protein 70. Cancer ResOctober 1, 2007 67; 9407.
Manzo SG et al. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012 Oct 15;72(20):5363-73.
Cai YY et al. Combined Effect of Curcumin and Triptolide on an Ovarian Cancer Cell Line. Asian Pac J Cancer Prev, 14 (7), 4267-4271
Chen H et al. Dihydroartemisinin inhibits growth of pancreatic cancer cells in vitro and in vivo. Anticancer Drugs. 2009 Feb;20(2):131-40.
Matrine inhibits proliferation and induces apoptosis of pancreatic cancer cells in vitro and in vivo. Biol Pharm Bull. 2010;33(10):1740-5.
Saruc M, Standop S, Standop J, Nozawa F, Itami A, Pandey KK, Batra SK, Gonzalez NJ, Guesry P, Pour PM. Pancreatic Enzyme Extract Improves Survival in Murine Pancreatic Cancer Pancreas, 2004; 28(4):401-412.
Gonzalez NJ, Isaacs LL. Evaluation of Pancreatic Proteolytic Enzyme Treatment of Adenocarcinoma of the Pancreas, With Nutrition and Detoxification Support Nutrition and Cancer 1999; 33(2): 117-124.
Benavides MA et al. L-Methionine inhibits growth of human pancreatic cancer cells. Anticancer Drugs. 2014 Feb;25(2):200-3.
Benavides MA et al. Suppression by L-methionine of cell cycle progression in LNCaP and MCF-7 cells but not benign cells. Anticancer Res. 2010 Jun;30(6):1881-5.
Benavides MA et al. Methionine inhibits cellular growth dependent on the p53 status of cells. Am J Surg. 2007 Feb;193(2):274-83.
Berkson BM, Rubin DM, Berkson AJ. The long-term survival of a patient with pancreatic cancer with metastases to the liver after treatment with the intravenous alpha-lipoic acid/low-dose naltrexone protocol. Integr Cancer Ther. 2006 Mar;5(1):83-9.
Berkson BM, Rubin DM, Berkson AJ. Revisiting the ALA/N (alpha-lipoic acid/low-dose naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: a report of 3 new cases. Integr Cancer Ther. 2009 Dec;8(4):416-22.
Shen W et al. Protective effects of R-alpha-lipoic acid and acetyl-L-carnitine in MIN6 and isolated rat islet cells chronically exposed to oleic acid. J Cell Biochem. 2008 Jul 1;104(4):1232-43.
Guais A et al. Adding a combination of hydroxycitrate and lipoic acid (METABLOC™) to chemotherapy improves effectiveness against tumor development: experimental results and case report. Invest New Drugs. 2012 Feb;30(1):200-11.
Zachar Z et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl). 2011 Nov;89(11):1137-48.
Tian YF, He CT, Chen YT, Hsieh PS. Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats. World J Gastroenterol. 2013 May 14;19(18):2761-71.
Tian YF et al. α-Lipoic acid prevents mild portal endotoxaemia-induced hepatic inflammation and β cell dysfunction. Eur J Clin Invest. 2012 Jun;42(6):637-48.
Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: amelioration by the antioxidant, alpha-lipoic acid. Am J Physiol Regul Integr Comp Physiol. 2010 May;298(5):R1343-50.
Poh ZX, Goh KP. A current update on the use of alpha lipoic acid in the management of type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2009 Dec;9(4):392-8.
Lee BW et al. Dose-related cytoprotective effect of alpha-lipoic acid on hydrogen peroxide-induced oxidative stress to pancreatic beta cells. Free Radic Res. 2009 Jan;43(1):68-77.
Karatug A, Bolkent S. The potential role of combined antioxidant treatment on pancreas of STZ-diabetic mice. Exp Toxicol Pathol. 2013 Mar;65(3):255-62.
Bulut NE et al. Beneficial effects of alpha lipoic acid on cerulein-induced experimental acute pancreatitis in rats. Ulus Travma Acil Cerrahi Derg. 2011 Sep;17(5):383-9.
Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. Eur J Pharmacol. 2010 Sep 25;643(2-3):289-96.
Le A, Rajeshkumar NV, Maitra A, Dang CV. Conceptual framework for cutting the pancreatic cancer fuel supply. Clin Cancer Res. 2012 Aug 15;18(16):4285-90.
Son J et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013 Apr 4;496(7443):101-5.
Savarese DM et al. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev 2003 29(6):501-13.
Yoshida S et al. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann surg 1998; 227(4):485-91.
Kuhn KS et al. Glutamine as an indispensable nutrient in oncology: experimental and clinical evidence. Eur J Nutr 2010; 48(4):197-210.