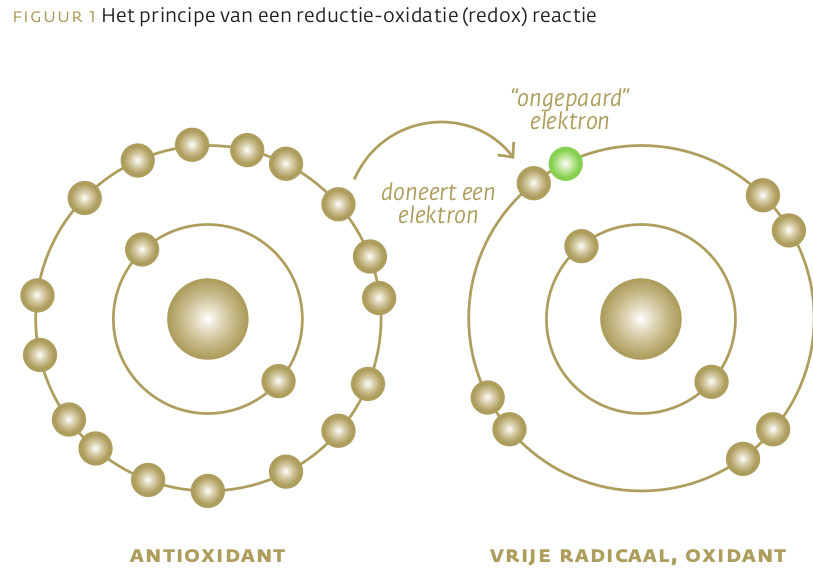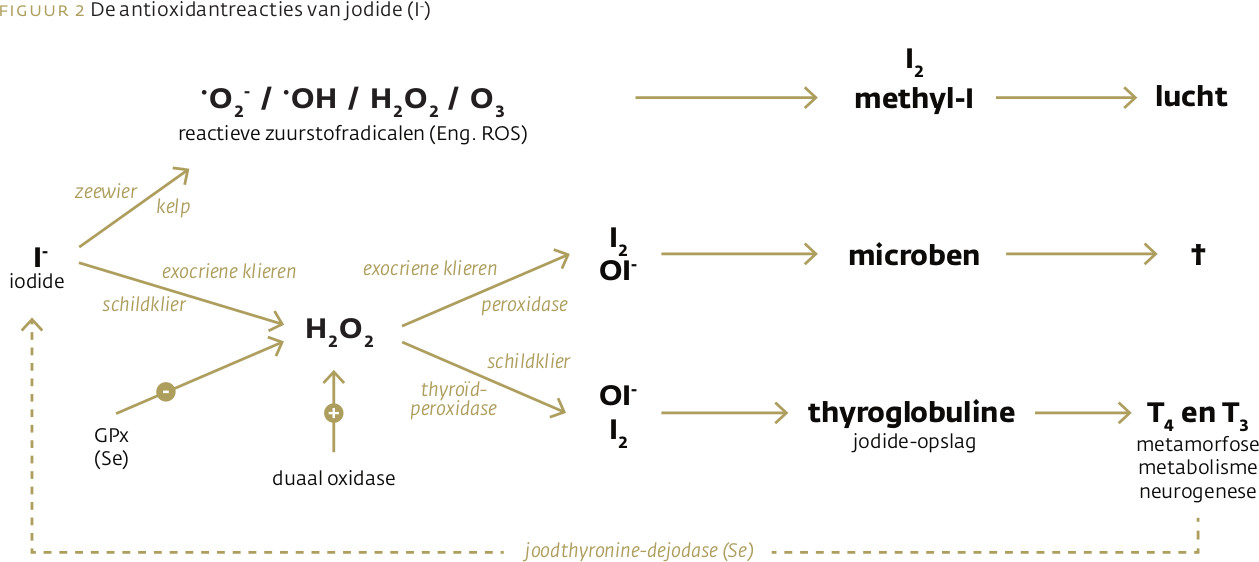
Jodide (I-) wordt niet alleen gebruikt voor de synthese van schildklierhormoon. Het eerste gebruik in de evolutie was als antioxidant en als antimicrobieel middel. De synthese van schildklierhormoon is een evolutionair recente modificatie. Zeewier gebruikt een elektron van jodide voor de detoxificatie van belagende zuurstofradicalen. Via het jodide/peroxidase/waterstof-partnersysteem kan een elektron van jodide worden overgeheveld op waterstofperoxide voor de synthese van moleculair jodium (I2) en hypojodiet (OI-) ten behoeve van de bestrijding van micro-organismen in exocriene klieren, maag-darmslijmvlies en longen. Met ditzelfde systeem vindt in de schildklier jodering plaats van thyroglobuline als eerste stap in de synthese van schildklierhormoon. Inzicht in het samenspel tussen het jodide/peroxidase/waterstof-partnersysteem en selenium is van belang voor het begrip van schildklierauto-immuunziektes en kanker van de schildklier, maag, borstklier en prostaat. De huidige jodiumstatus in Nederland is zorgelijk. Dit bedreigt niet alleen de schildklierhormoonsynthese maar vooral de functies van jodide als antioxidant en antimicrobieel middel. De huidige aanbevolen dagelijkse hoeveelheid (ADH) van jodium is gebaseerd op de jodiumbehoefte van de schildklier en het voorkómen van een vergrote schildklier. De absolute bovengrens is gebaseerd op een waarschijnlijk kleine groep van mensen met een defecte schildklier-autoregulatie. Er is geen rekening gehouden met de oerfuncties van jodide en het voorkómen van jodiumtoxiciteit door de jodiuminname te koppelen aan de seleniumstatus.
Beste bezoeker, u heeft geen toegang.
Enkel (web)abonnees hebben toegang tot tijdschriftartikelen. Het webabonnement is nog in de maak.
U kunt zich wel alvast (gratis) registreren en tal van andere webartikelen raadplegen!
Auteur
Verschenen in
Referenties
Venturi S, Venturi M. Iodide, thyroid and stomach carcinogenesis: evolutionary story of a primitive antioxidant?. Eur J Endocrinol. 1999;140(4):371‐372. doi:10.1530/eje.0.1400371
Winkler R. Iodine—A Potential Antioxidant and the Role of Iodine/Iodide in Health and Disease. Natural Science, 2015, 7, 548-557, Published Online November 2015 in SciRes. http://www.scirp.org/journal/ns, doi.org/10.4236/ns.2015.712055
file:///C:/Users/Eigenaar/Downloads/Iodine-A_Potential_Antioxidant_and_the_Role_of_Iod.pdf
De la Vieja A, Santisteban P. Role of iodide metabolism in physiology and cancer. Endocr Relat Cancer. 2018;25(4):R225‐R245. doi:10.1530/ERC-17-0515
Venturi S. Evolutionary significance of iodine. Current Chemical Biology, 2011, 5, 155-162.
file:///C:/Users/Eigenaar/Downloads/EvolutionarySignificanceodIodine-CCB201153.pagg.155-162.pdf
Blankenship RE. Early evolution of photosynthesis. Plant Physiol. 2010;154(2):434‐438. doi:10.1104/pp.110.161687
https://pubmed.ncbi.nlm.nih.gov/20921158/?from_term=Blankenship+Plant+Physiology+2010&from_pos=1
Gonzales J, Tymon T, Küpper FC, Edwards MS, Carrano CJ. The potential role of kelp forests on iodine speciation in coastal seawater [published correction appears in PLoS One. 2017 Dec 7;12 (12 ):e0189559]. PLoS One. 2017;12(8):e0180755. Published 2017 Aug 11. doi:10.1371/journal.pone.0180755
https://pubmed.ncbi.nlm.nih.gov/28800586/?from_term=Gonzales+PLOS+ONE+2017&from_pos=2
Küpper FC, Carpenter LJ, McFiggans GB, et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci U S A. 2008;105(19):6954‐6958. doi:10.1073/pnas.0709959105
Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004;14(10):836‐841. doi:10.1089/thy.2004.14.836
Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res. 2011;4:14. Published 2011 Oct 5. doi:10.1186/1756-6614-4-14
McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance [published correction appears in Clin Microbiol Rev 2001 Jan;14(1):227]. Clin Microbiol Rev. 1999;12(1):147‐179.
Brown-Grant K. Extrathyroidal Iodide Concentrating Mechanisms. Phyiol. Rev. 1961;41:189-213. doi.org/10.1152/physrev.1961.41.1.189
https://journals.physiology.org/doi/abs/10.1152/physrev.1961.41.1.189?journalCode=physrev
Aceves C, Anguiano B, Delgado G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid. 2013;23(8):938‐946. doi:10.1089/thy.2012.0579
https://pubmed.ncbi.nlm.nih.gov/23607319/?from_term=Aceves+Thyroid.+2013&from_pos=1
Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu Rev Physiol. 2017;79:261‐289. doi:10.1146/annurev-physiol-022516-034125
Sarr D, Tóth E, Gingerich A, Rada B. Antimicrobial actions of dual oxidases and lactoperoxidase. J Microbiol. 2018;56(6):373‐386. doi:10.1007/s12275-018-7545-1
Venturi S, Venturi M. Iodine in evolution of salivary glands and in oral health. Nutr Health. 2009;20(2):119‐134. doi:10.1177/026010600902000204
Venturi S, Begin ME. Thyroid Hormone, Iodine and Human Brain Evolution. Chapter 6. Human Brain Evolution. Book Editor(s): Stephen C. Cunnane Kathlyn M. Stewart. doi.org/10.1002/9780470609880.ch6 05 May 2010
https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470609880.ch6
Aceves C, Anguiano B, Delgado G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid. 2013;23(8):938‐946. doi:10.1089/thy.2012.0579
https://pubmed.ncbi.nlm.nih.gov/23607319/?from_term=Aceves+Thyroid.+2013&from_pos=1
Ravera S, Reyna-Neyra A, Ferrandino G, Amzel LM, Carrasco N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu Rev Physiol. 2017;79:261‐289. doi:10.1146/annurev-physiol-022516-034125
Kaczor T. Iodine and Cancer. A summary of the evidence to date. Nautural Medicine Journal June 2014 Vol. 6 Issue 6
https://www.naturalmedicinejournal.com/journal/2014-06/iodine-and-cancer
Venturi S, Donati FM, Venturi A, Venturi M. Environmental iodine deficiency: A challenge to the evolution of terrestrial life?. Thyroid. 2000;10(8):727‐729. doi:10.1089/10507250050137851
https://pubmed.ncbi.nlm.nih.gov/11014322/?from_term=Venturi+Thyroid+2000&from_pos=2
Dunford HB. Peroxidase-catalyzed halide ion oxidation. Redox Rep. 2000;5(4):169‐171. doi:10.1179/135100000101535708
Day BJ, Bratcher PE, Chandler JD, et al. The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host. Free Radic Biol Med. 2020;146:324‐332. doi:10.1016/j.freeradbiomed.2019.11.016
https://pubmed.ncbi.nlm.nih.gov/31740228/
Patel U, Gingerich A, Widman L, Sarr D, Tripp RA, Rada B. Susceptibility of influenza viruses to hypothiocyanite and hypoiodite produced by lactoperoxidase in a cell-free system. PLoS One. 2018;13(7):e0199167. Published 2018 Jul 25. doi:10.1371/journal.pone.0199167
Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32(5):670‐693. doi:10.1210/er.2011-0007
Kwakkel J, Fliers E, Boelen A. Illness-induced changes in thyroid hormone metabolism: focus on the tissue level. Neth J Med. 2011;69(5):224‐228.
Hendriksen M, Etemad Z, van den Bogaard CHM, van der A DL. Zout-, jodium- en kaliuminname 2015. Voedingsstatusonderzoek bij volwassenen uit Doetinchem. 04-10-2016; RIVM Rapport 2016-0081.
https://www.rivm.nl/bibliotheek/rapporten/2016-0081.pdfD
Besluit van 13 juni 2008, houdende wijziging van het Warenwetbesluit Toevoeging micro- voedingsstoffen aan levensmiddelen, inzake het toevoegen van jodium. Staatsblad. 2008;257:1-5.
https://zoek.officielebekendmakingen.nl/stb-2008-257.html
Stoutjesdijk E, Schaafsma A, Dijck-Brouwer DAJ, Muskiet FAJ. Iodine status during pregnancy and lactation: a pilot study in the Netherlands. Neth J Med. 2018;76(5):210‐217.
Cavalieri RR. Iodine metabolism and thyroid physiology: current concepts. Thyroid. 1997;7(2):177‐181. doi:10.1089/thy.1997.7.177
Triggiani V, Tafaro E, Giagulli VA, et al. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disord Drug Targets. 2009;9(3):277‐294. doi:10.2174/187153009789044392
Raiten DJ, Sakr Ashour FA, Ross AC, et al. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr. 2015;145(5):1039S‐1108S. doi:10.3945/jn.114.194571
Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12(4):1181. Published 2020 Apr 23. doi:10.3390/nu12041181
https://pubmed.ncbi.nlm.nih.gov/32340216/?from_term=Calder+Nutrients+2020&from_pos=1
Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1):236. Published 2020 Jan 16. doi:10.3390/nu12010236
https://pubmed.ncbi.nlm.nih.gov/31963293/?from_term=Gombart+Nutrients+2020&from_pos=1
VoedingsCentrum Jodium.
https://www.voedingscentrum.nl/encyclopedie/jodium.aspx
Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001.
https://www.nap.edu/initiative/panel-on-micronutrients
Scientific Opinion on Dietary Reference Values for iodine1 EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2,3 European Food Safety Authority (EFSA), Parma, Italy. EFSA Journal 2014;12(5):3660
https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3660
Scientific Committee on Food Scientific Panel on Dietetic Products, Nutrition and Allergies TOLERABLE UPPER INTAKE LEVELS FOR VITAMINS AND MINERALS European Food Safety Authority February 2006. ISBN: 92-9199-014-0
http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf
Miller DW. Extrathyroidal Benefits of Iodine. Journal of American Physicians and Surgeons Volume 11 Number 4 Winter 2006 106-110
https://www.jpands.org/vol11no4/millerd.pdf
Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136‐142. doi:10.1038/nrendo.2013.251
Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: Radiographic iodinated contrast media-induced thyroid dysfunction. J Clin Endocrinol Metab. 2015;100(2):376‐383. doi:10.1210/jc.2014-3292
Katagiri R, Yuan X, Kobayashi S, Sasaki S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS One. 2017;12(3):e0173722. Published 2017 Mar 10. doi:10.1371/journal.pone.0173722
Leung A, Pearce EN, Braverman LE. Role of iodine in thyroid physiology. Expert Rev Endocrinol Metab. 2010;5(4):593‐602. doi:10.1586/eem.10.40
Oster O, Schmiedel G, Prellwitz W. The organ distribution of selenium in German adults. Biol Trace Elem Res. 1988;15:23‐45. doi:10.1007/BF02990125
https://pubmed.ncbi.nlm.nih.gov/2484520/?from_term=Oster+Biol.+Trace+Elem.+Res.+1988&from_pos=1
Zhou BF, Stamler J, Dennis B, et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17(9):623‐630. doi:10.1038/sj.jhh.1001605
https://pubmed.ncbi.nlm.nih.gov/13679952/
Yoneyama S, Miura K, Itai K, et al. Dietary intake and urinary excretion of selenium in the Japanese adult population: the INTERMAP Study Japan. Eur J Clin Nutr. 2008;62(10):1187‐1193. doi:10.1038/sj.ejcn.1602842
https://pubmed.ncbi.nlm.nih.gov/17622257/
Song Y, Driessens N, Costa M, et al. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007;92(10):3764‐3773. doi:10.1210/jc.2007-0660
Winther KH, Rayman MP, Bonnema SJ, Hegedüs L. Selenium in thyroid disorders - essential knowledge for clinicians. Nat Rev Endocrinol. 2020;16(3):165‐176. doi:10.1038/s41574-019-0311-6
Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78(1):34‐44. doi:10.1017/S0029665118001192
Wang B, He W, Li Q, et al. U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur J Endocrinol. 2019;181(3):255‐266. doi:10.1530/EJE-19-0212
https://pubmed.ncbi.nlm.nih.gov/31252413/?from_term=Wang+Eur+J+Endocrinol.+2019&from_pos=3
Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256‐1268. doi:10.1016/S0140-6736(11)61452-9
Rayman MP. Selenium intake, status, and health: a complex relationship. Hormones (Athens). 2020;19(1):9‐14. doi:10.1007/s42000-019-00125-5
McCann JC, Ames BN. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011;25(6):1793‐1814. doi:10.1096/fj.11-180885
Guastamacchia E, Giagulli VA, Licchelli B, Triggiani V. Selenium and Iodine in Autoimmune Thyroiditis. Endocr Metab Immune Disord Drug Targets. 2015;15(4):288‐292. doi:10.2174/1871530315666150619094242
Benstoem C, Goetzenich A, Kraemer S, et al. Selenium and its supplementation in cardiovascular disease--what do we know?. Nutrients. 2015;7(5):3094‐3118. Published 2015 Apr 27. doi:10.3390/nu7053094
Avery JC, Hoffmann PR. Selenium, Selenoproteins, and Immunity. Nutrients. 2018;10(9):1203. Published 2018 Sep 1. doi:10.3390/nu10091203
Ruiz-Núñez B, Tarasse R, Vogelaar EF, Janneke Dijck-Brouwer DA, Muskiet FAJ. Higher Prevalence of "Low T3 Syndrome" in Patients With Chronic Fatigue Syndrome: A Case-Control Study. Front Endocrinol (Lausanne). 2018;9:97. Published 2018 Mar 20. doi:10.3389/fendo.2018.00097
Arthur JR, Beckett GJ, Mitchell JH. The interactions between selenium and iodine deficiencies in man and animals. Nutr Res Rev. 1999;12(1):55‐73. doi:10.1079/095442299108728910
Xu J, Yang XF, Guo HL, Hou XH, Liu LG, Sun XF. Selenium supplement alleviated the toxic effects of excessive iodine in mice. Biol Trace Elem Res. 2006;111(1-3):229‐238. doi:10.1385/BTER:111:1:229
Xu J, Liu XL, Yang XF, Guo HL, Zhao LN, Sun XF. Supplemental selenium alleviates the toxic effects of excessive iodine on thyroid. Biol Trace Elem Res. 2011;141(1-3):110‐118. doi:10.1007/s12011-010-8728-8
Thomson CD, Campbell JM, Miller J, Skeaff SA. Minimal impact of excess iodate intake on thyroid hormones and selenium status in older New Zealanders. Eur J Endocrinol. 2011;165(5):745‐752. doi:10.1530/EJE-11-0575
McGregor B. The Role of Selenium in Thyroid Autoimmunity: A Review. Journal of Restorative Medicine 2015; 4: 83-92.DOI 10.14200/jrm.2015.4.0102
https://journal.restorativemedicine.org/index.php/journal/article/view/61/99
Guastamacchia E, Giagulli VA, Licchelli B, Triggiani V. Selenium and Iodine in Autoimmune Thyroiditis. Endocr Metab Immune Disord Drug Targets. 2015;15(4):288‐292. doi:10.2174/1871530315666150619094242
Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid. 2016;26(12):1681‐1692. doi:10.1089/thy.2016.0256
https://pubmed.ncbi.nlm.nih.gov/27702392/?from_term=Wichman+Thyroid+2016&from_sort=date&from_pos=1
Köhrle J. Selenium and the thyroid. Curr Opin Endocrinol Diabetes Obes. 2015;22(5):392‐401. doi:10.1097/MED.0000000000000190
Valea A, Georgescu CE. Selenoproteins in human body: focus on thyroid pathophysiology. Hormones (Athens). 2018;17(2):183‐196. doi:10.1007/s42000-018-0033-5
https://pubmed.ncbi.nlm.nih.gov/29873029/?from_term=Valea+Hormones+2018&from_sort=date&from_pos=1
Cunnane SC, Crawford MA. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):17‐26. doi:10.1016/s1095-6433(03)00048-5
Cunnane SC. Survival of the Fattest: The Key to Human Brain Evolution. 1st ed. Singapore: World Scientific Publishing Company; 2005
https://www.amazon.com/Survival-Fattest-Human-Brain-Evolution/dp/9812561919
Orozco A, Valverde-R C, Olvera A, García-G C. Iodothyronine deiodinases: a functional and evolutionary perspective. J Endocrinol. 2012;215(2):207‐219. doi:10.1530/JOE-12-0258
Noahsen P, Kleist I, Larsen HM, Andersen S. Intake of seaweed as part of a single sushi meal, iodine excretion and thyroid function in euthyroid subjects: a randomized dinner study. J Endocrinol Invest. 2020;43(4):431‐438. doi:10.1007/s40618-019-01122-6